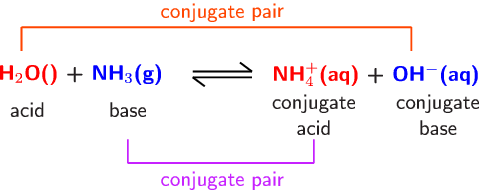
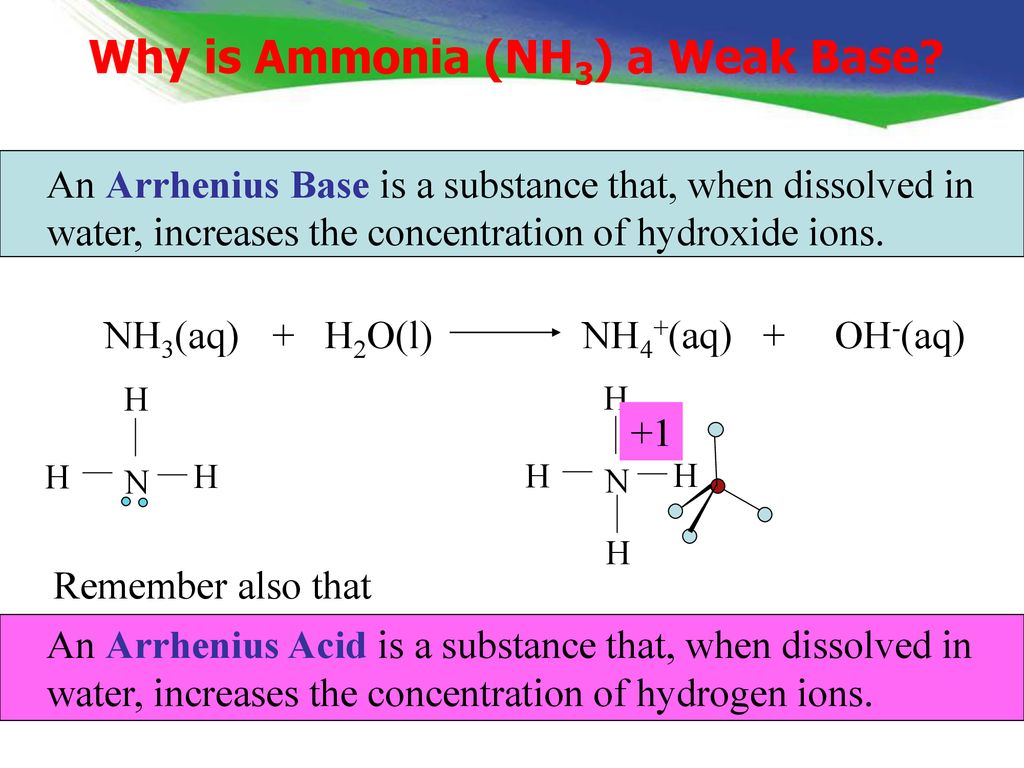
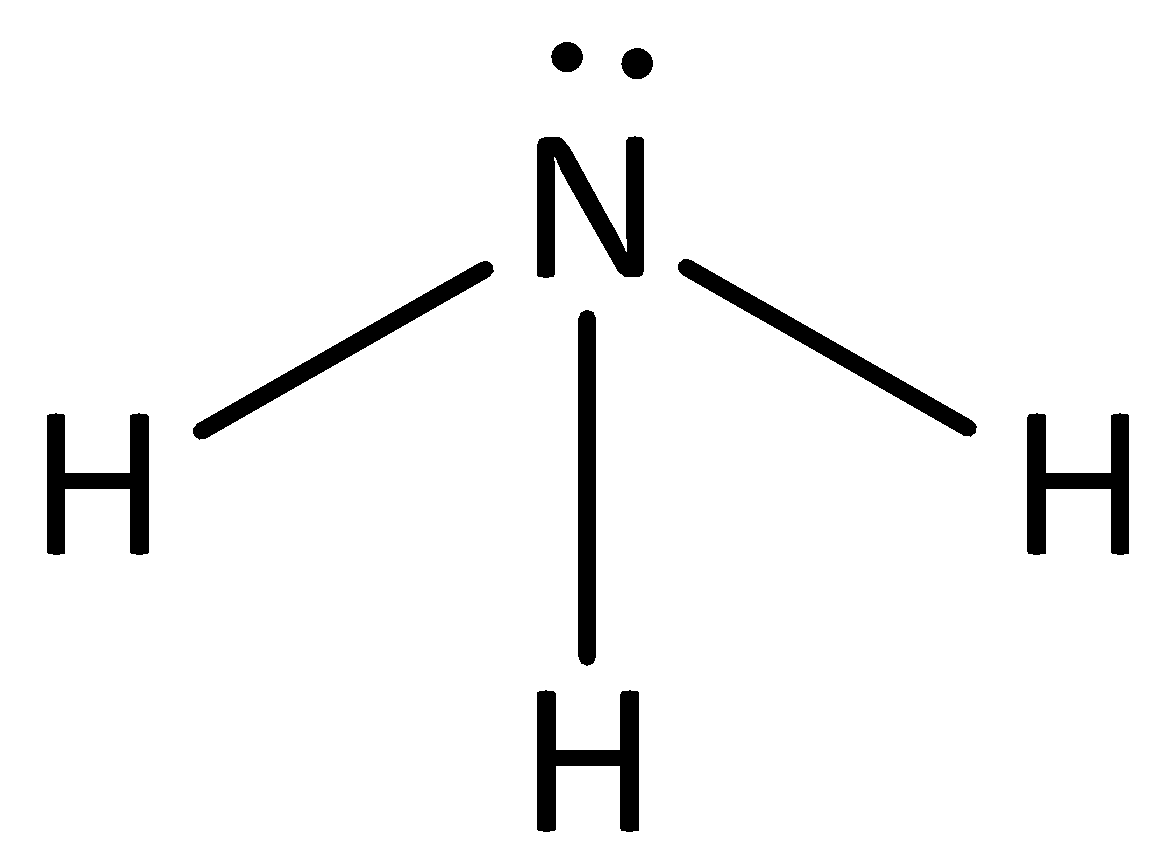
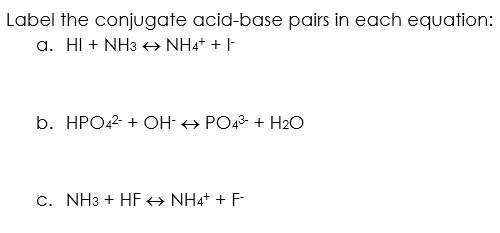Identify the conjugate acid-base pairs in this equilibrium. NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com
Why does boron trifluoride (BF3) act as a Lewis base and ammonia (NH3) acts as a Lewis acid? - Quora
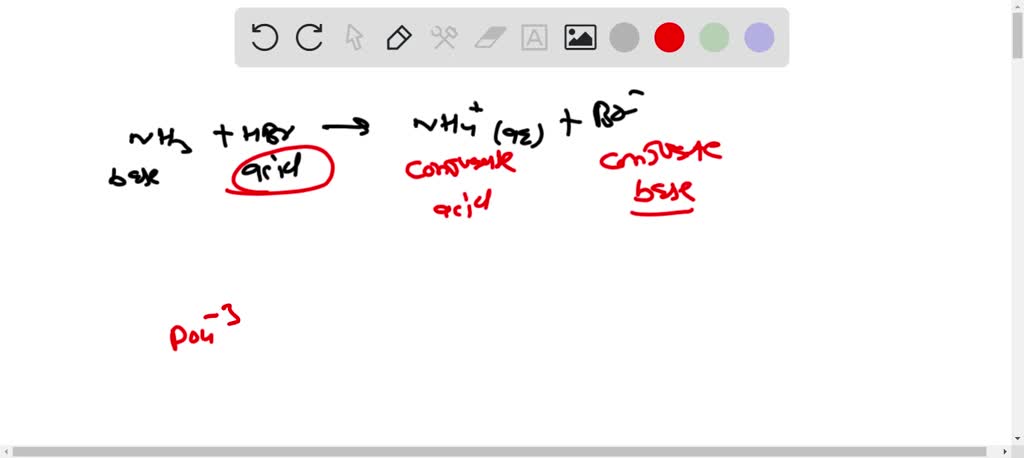
SOLVED: 16. Write an equation that shows the reaction of ammonia, NH3 with hydrobromic acid, HBr. Label the acid,the base, the conjugate acid, and the conjugate base. Write an equation that shows
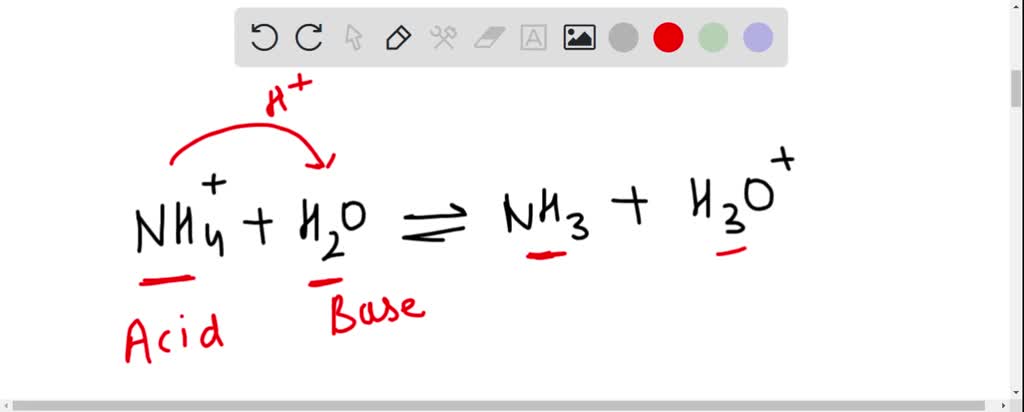
SOLVED: In the following reaction: NH4+ + H2O = NH3 + H3O+ A) H2O is a base and NH3 is its conjugate acid B) NH4+ is an acid and H20 is its

![Why does \\[N{H_3}\\] act as a Lewis base? Why does \\[N{H_3}\\] act as a Lewis base?](https://www.vedantu.com/question-sets/db470ea0-7477-412c-82d8-a4917a1132145281377342200076057.png)