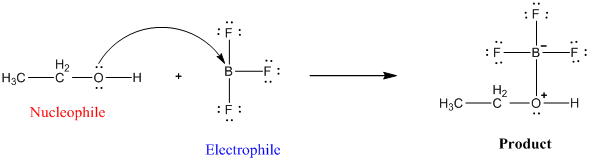
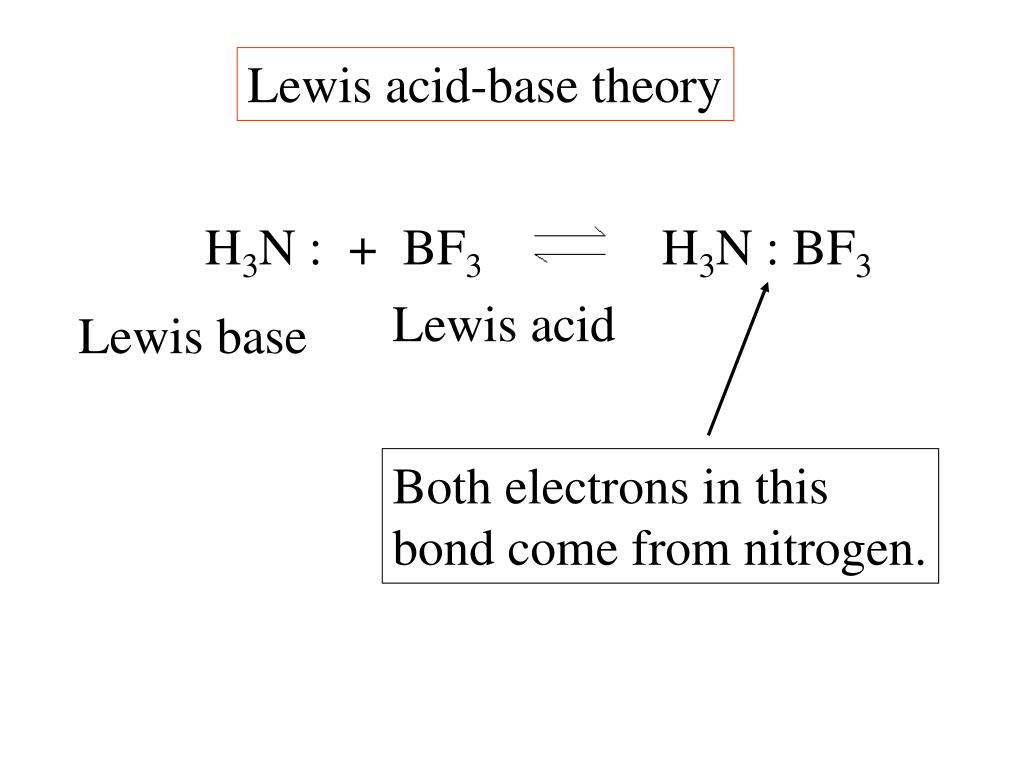
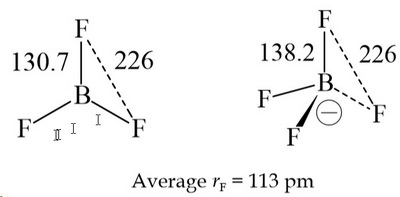
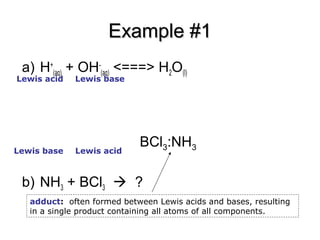
Welcome to Chem Zipper.com......: What are the order of extent back bonding, Lewis acid character and nucleophilicity of (BF3, BCl3, BBr3, BI3)boron trihalides?

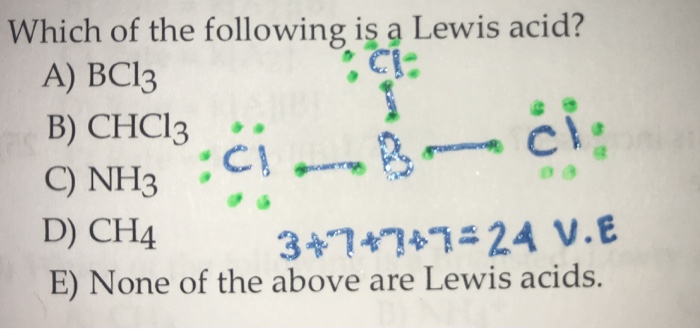
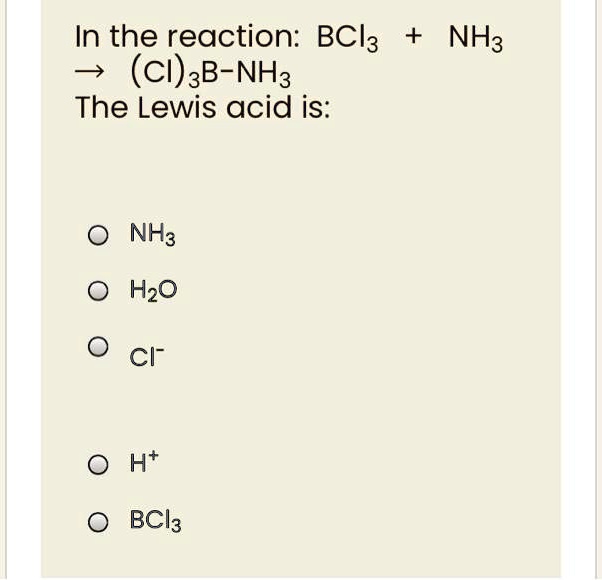
SOLVED: Identify the Lewis acids and Lewis bases: BCl3 +Ci = BCIA" The Lewis base is CI^ The Lewis acid is BCI3 SO2 + H20 = H2803 The Lewis base is H2O

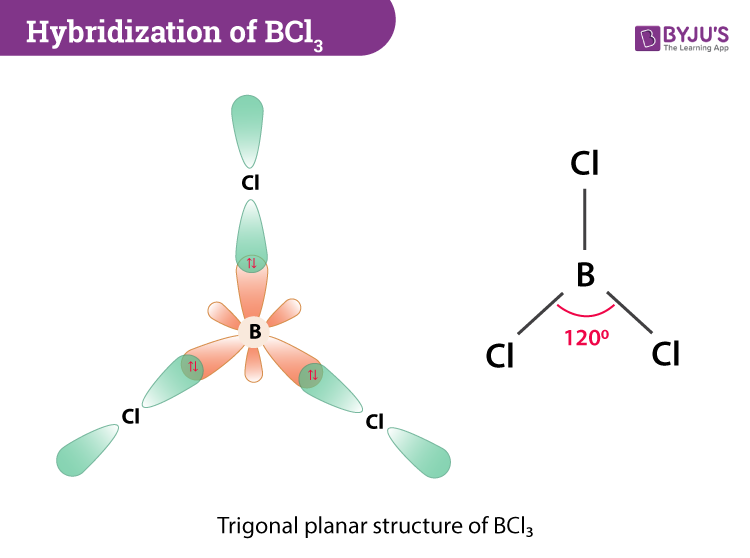
BCl3 lewis structure, molecular geometry, polar or nonpolar, hybridization, Bond angle in 2022 | Molecular geometry, Molecular, Vsepr theory

From σ- to π-Electrophilic Lewis Acids. Application to Selective Organic Transformations | The Journal of Organic Chemistry