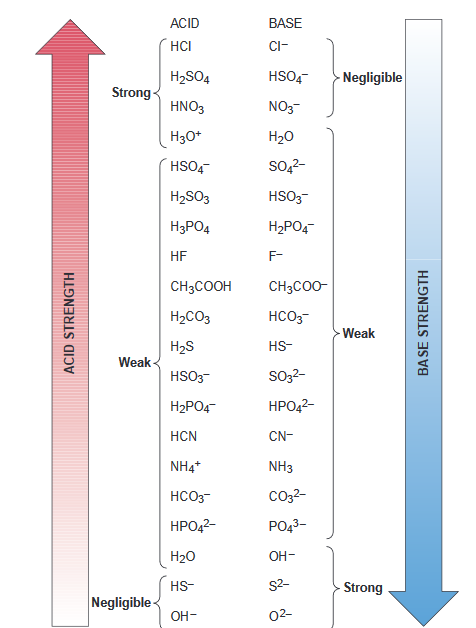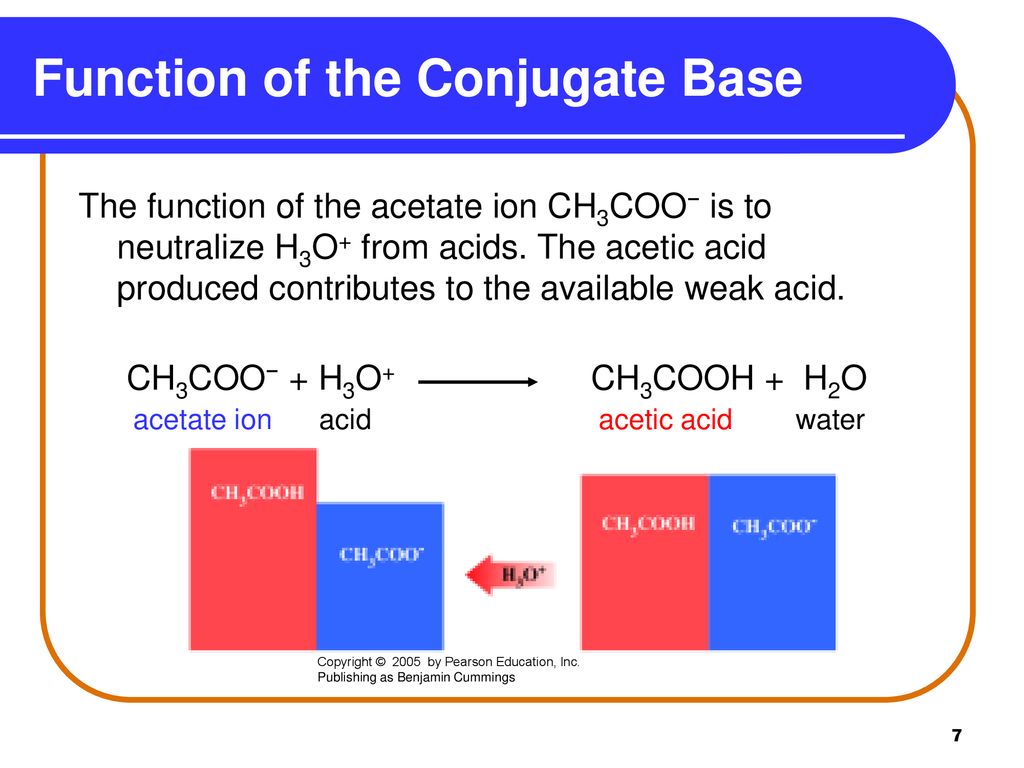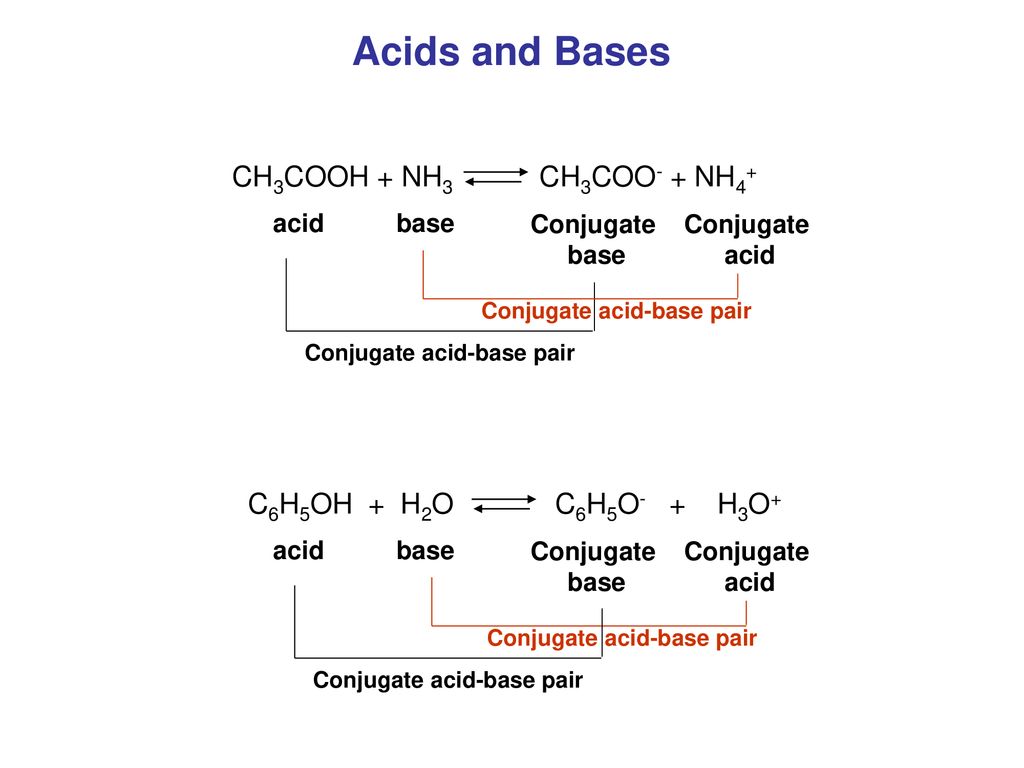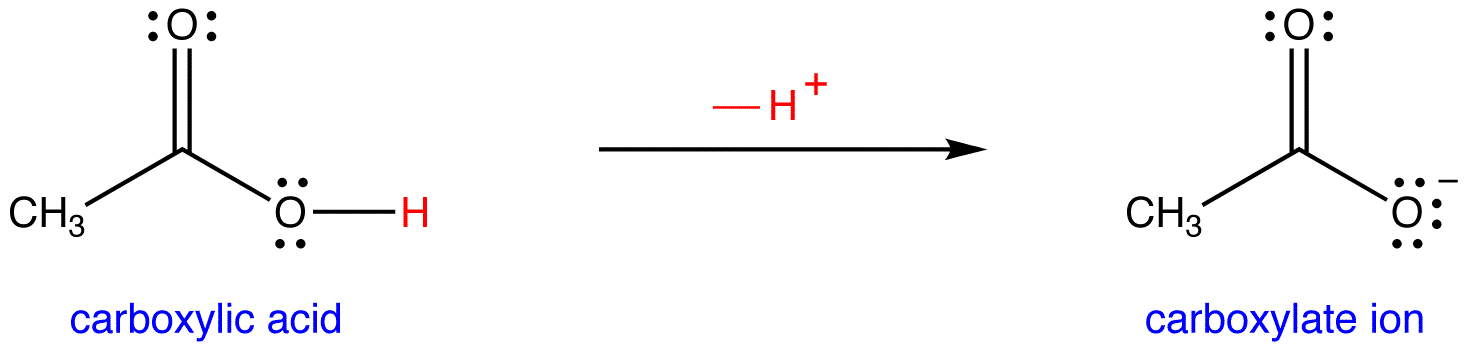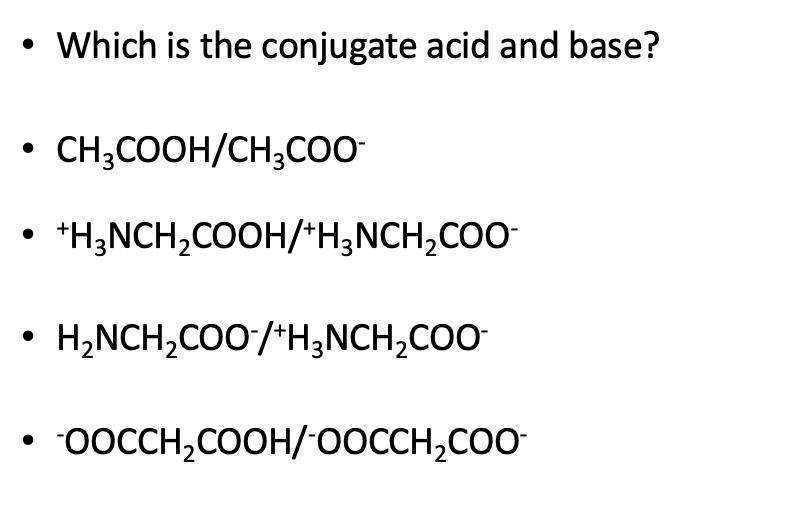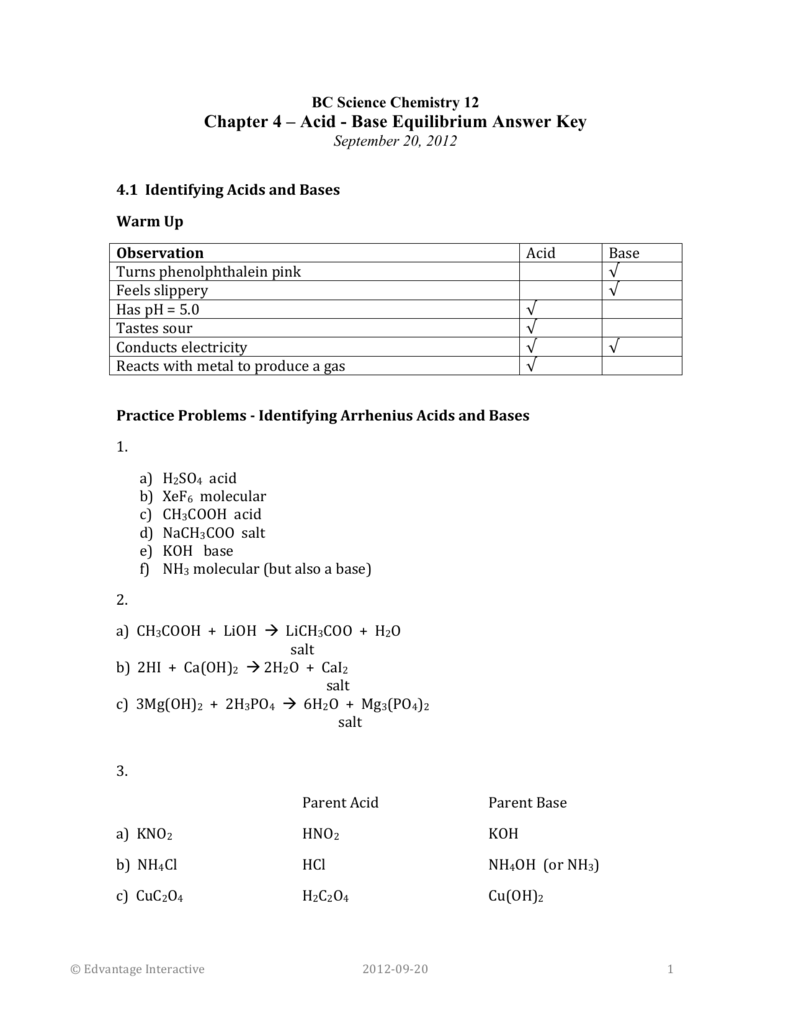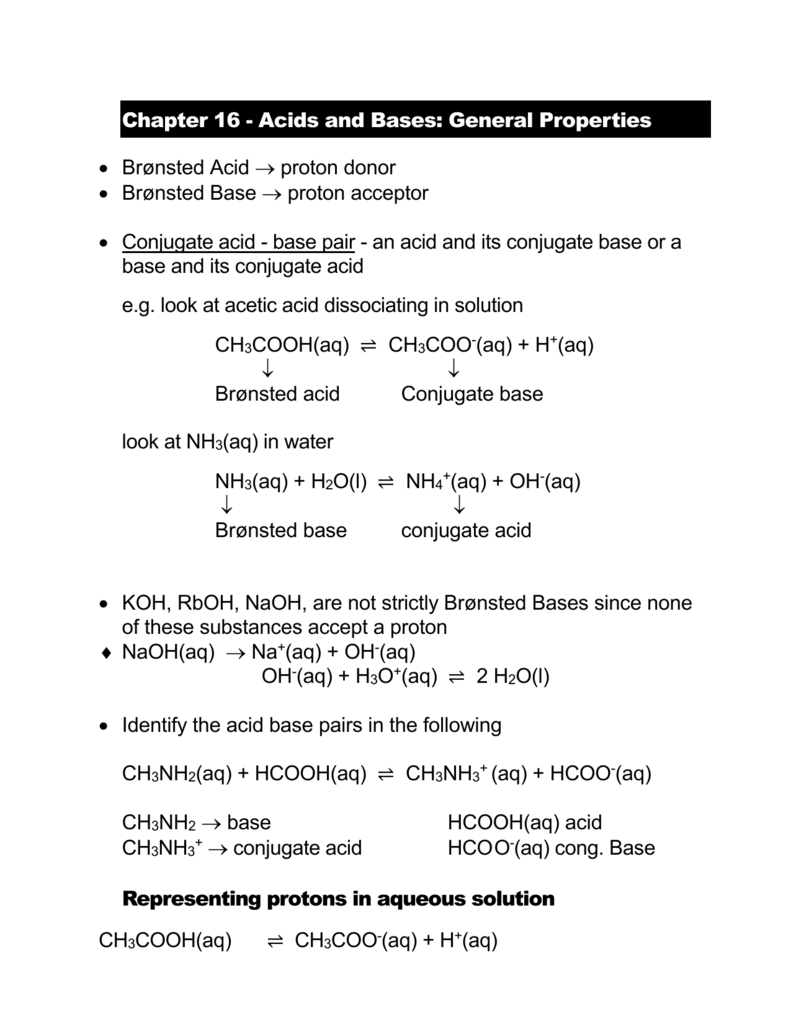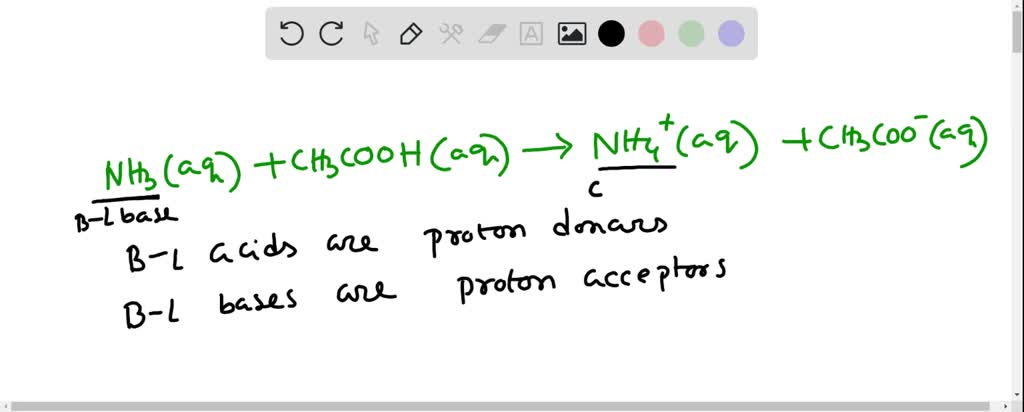
SOLVED: Identify the acid, base, conjugate acid and conjugate base in the following reactions: 1. NH3(aq) + CH3COOH(aq) —> NH4+(aq) + CH3COO- (aq)
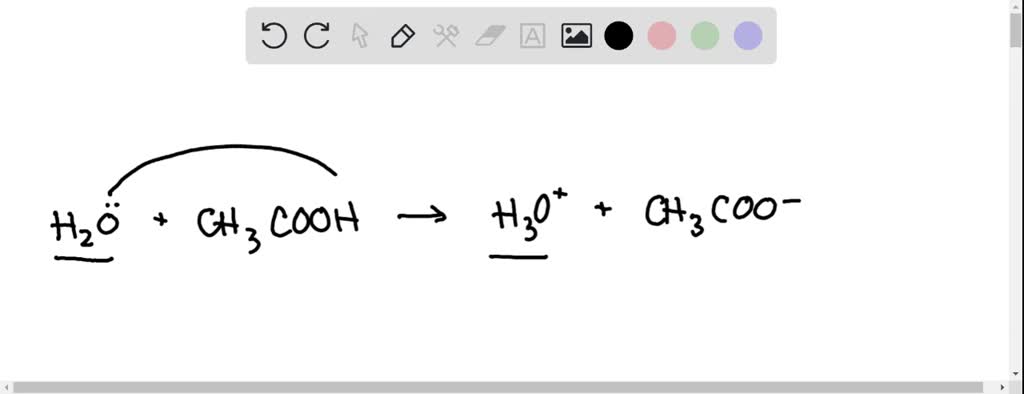
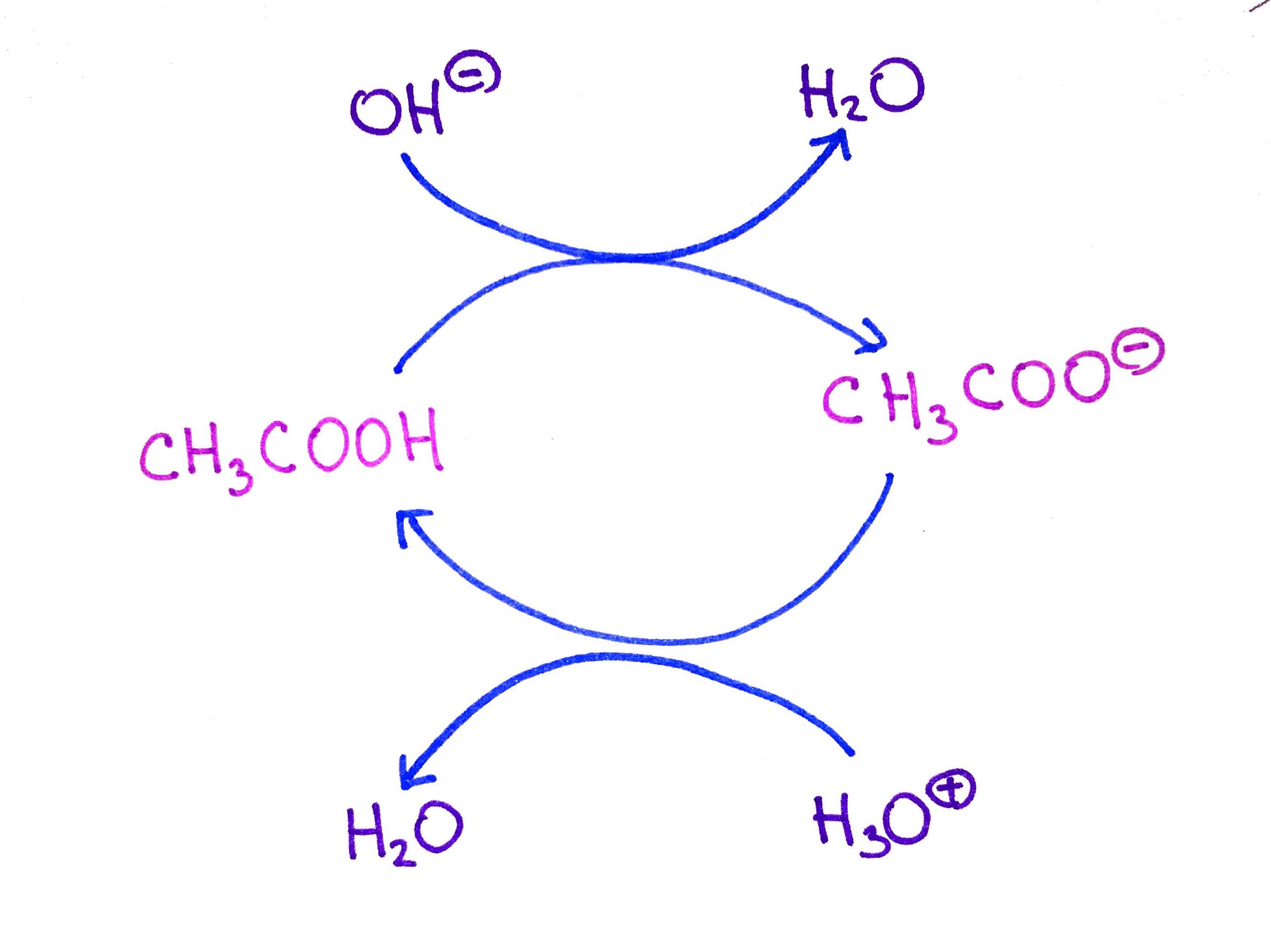
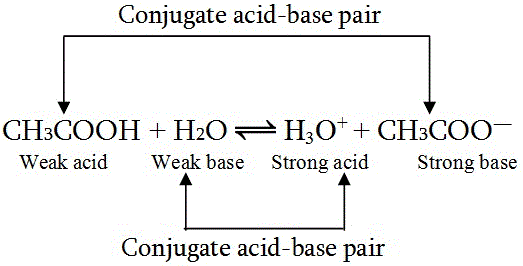
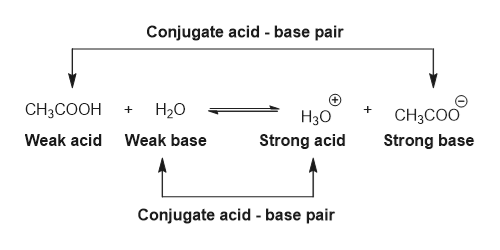
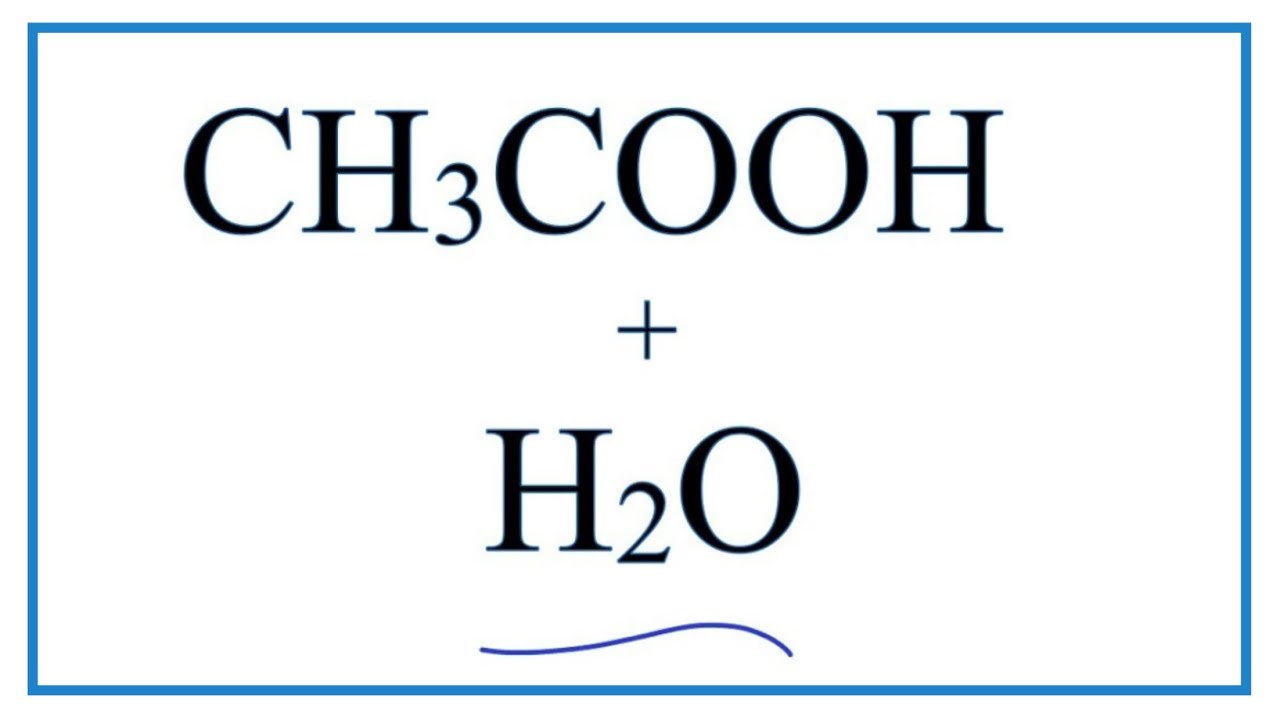
SOLVED: In the following chemical equation, identify the Bronsted-Lowry acid and the Bronsted-Lowry base H2O + CH3COOH CH3COO− + H3O+