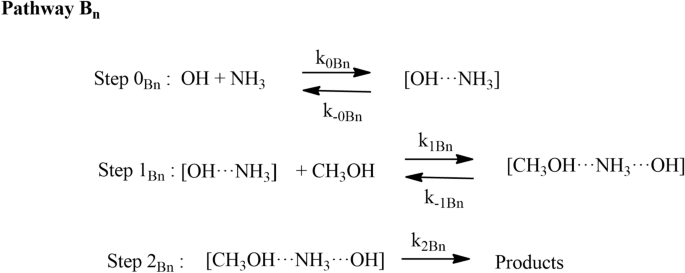
Effect of ammonia and water molecule on OH + CH3OH reaction under tropospheric condition | Scientific Reports
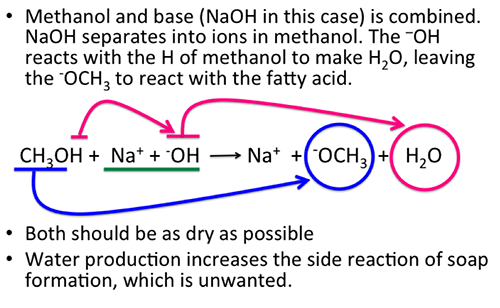
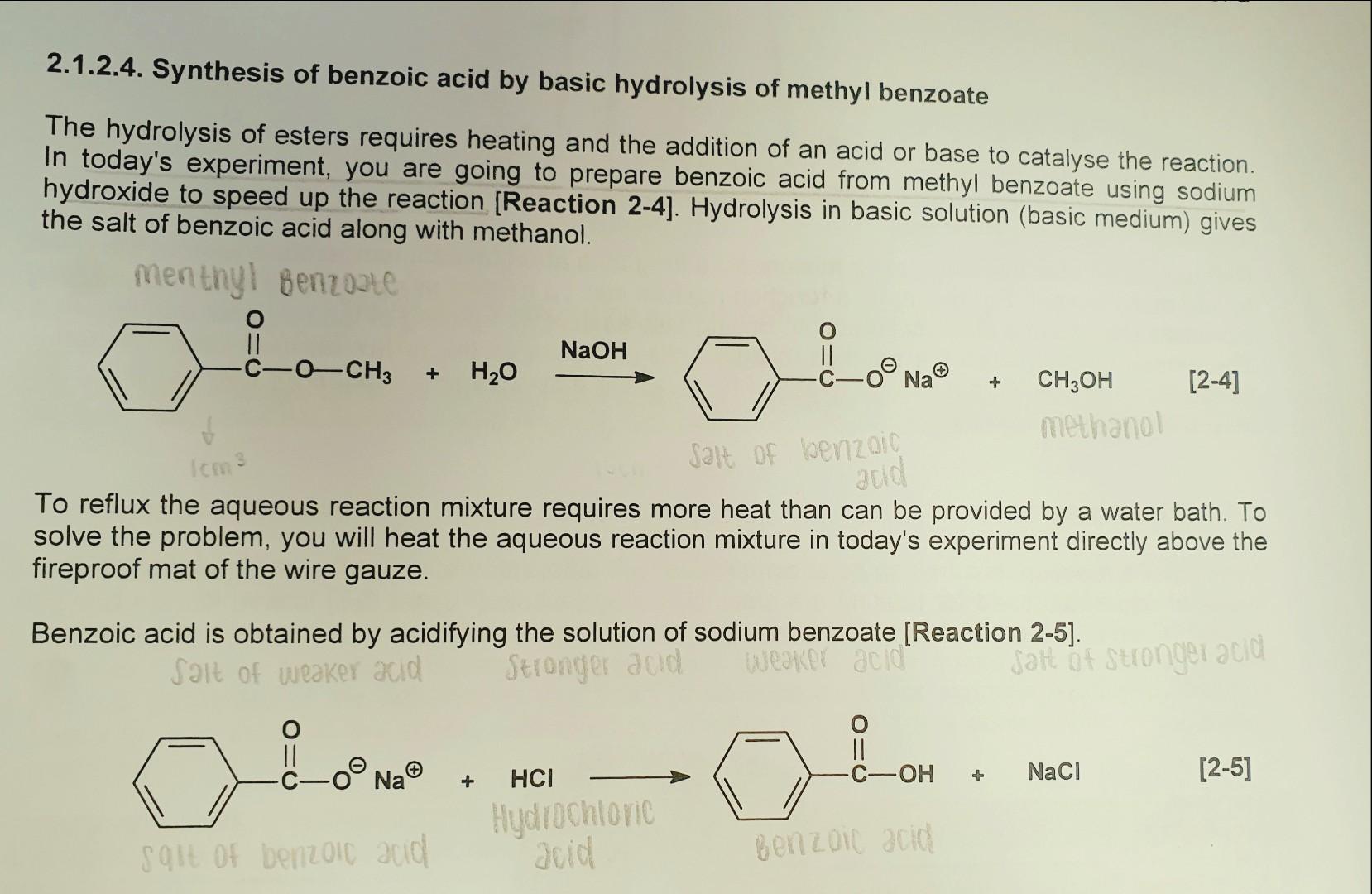
9.2 The Reaction of Biodiesel: Transesterification | EGEE 439: Alternative Fuels from Biomass Sources

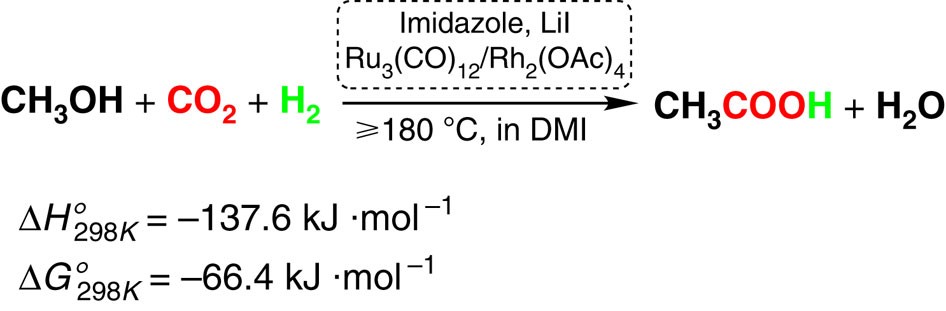
Catalysts | Free Full-Text | Selective Methanol Oxidation to Green Oxygenates—Catalyst Screening, Reaction Kinetics and Performance in Fixed-Bed and Membrane Reactors

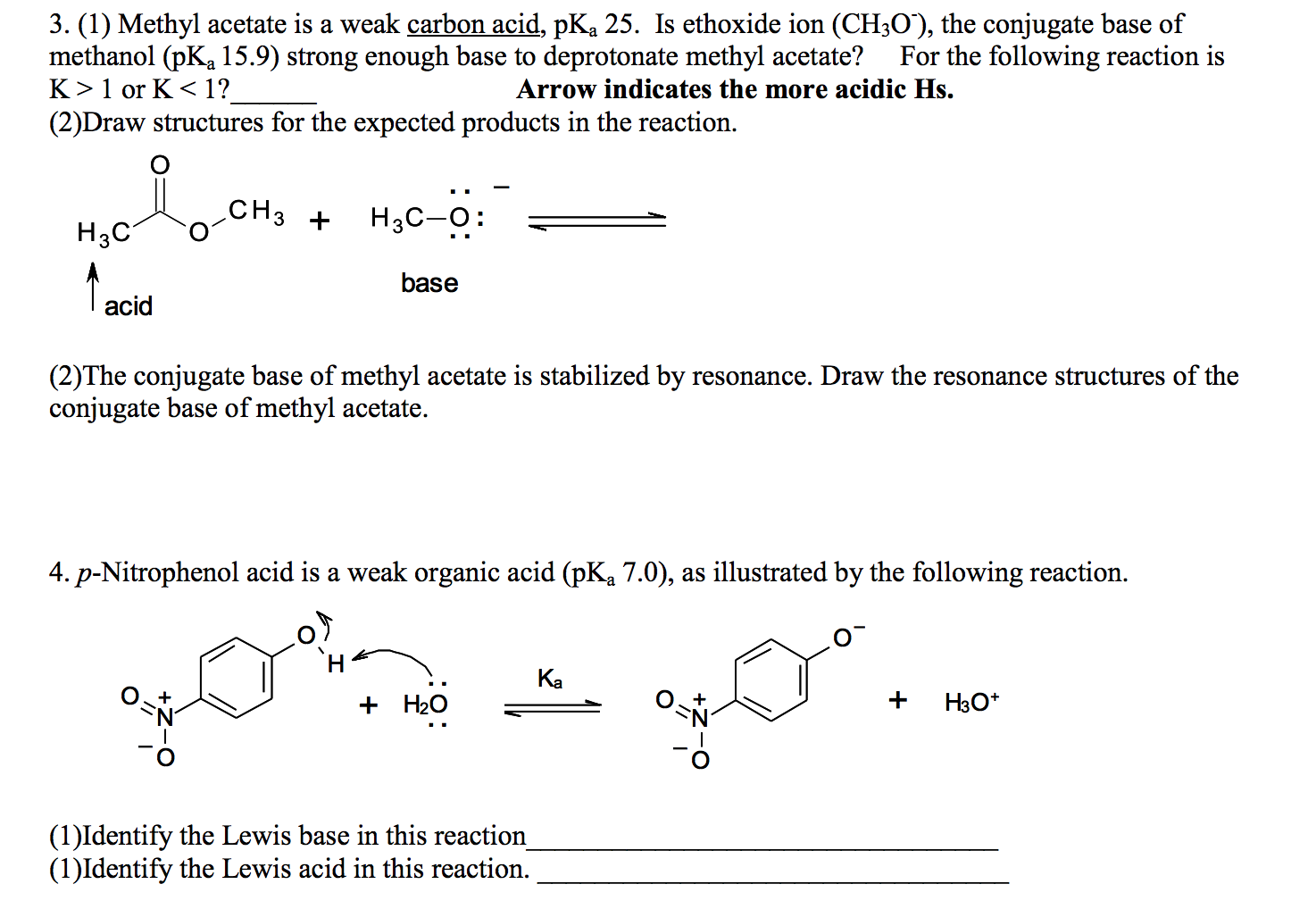
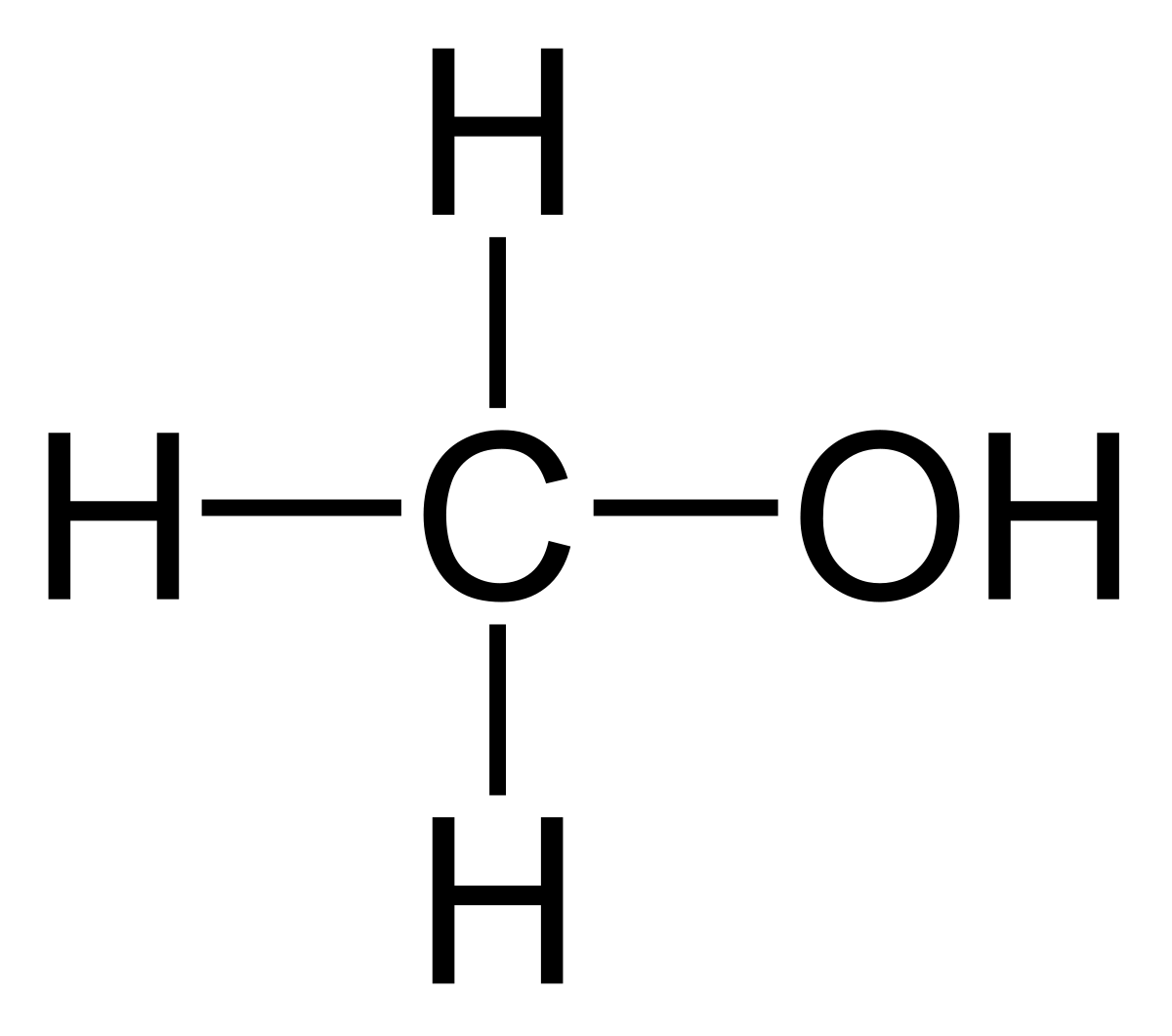
Can methanol be classified as an Arrhenius acid? To my knowledge it does not want the H from the O--H bond to dissociate : r/Mcat

SOLVED:Alcohols can act either as weak acids or as weak bases, just as water can. Show the reaction of methanol, CH3 OH, with a strong acid such as HCl and with a
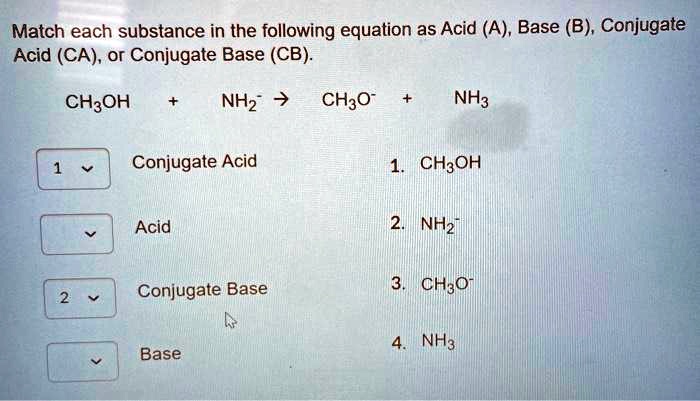
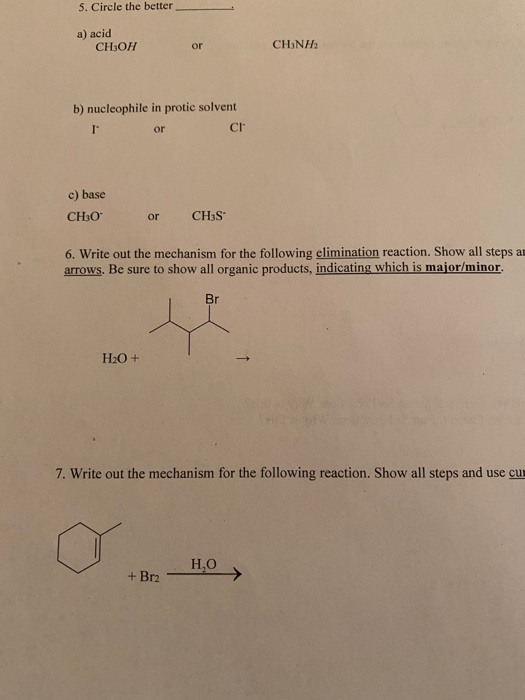
SOLVED: Match each substance in the following equation as Acid (A), Base (B), Conjugate Acid (CA), or Conjugate Base (CB) CH3OH NH2 CH3O" NH3 Conjugate Acid CHzOH Acid NH2 Conjugate Base CH3o-
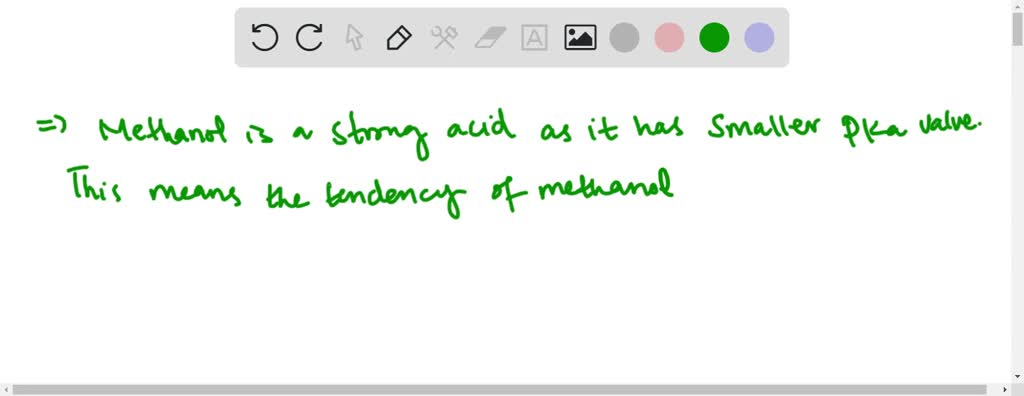
SOLVED:Does methanol behave as an acid or a base when it reacts with methylamine? (Hint: See page 55 for the structures of methanol and methylamine.)