Model‐Based Overpotential Deconvolution, Partial Impedance Spectroscopy, and Sensitivity Analysis of a Lithium‐Ion Cell with Blend Cathode - Quarti - 2021 - Energy Technology - Wiley Online Library
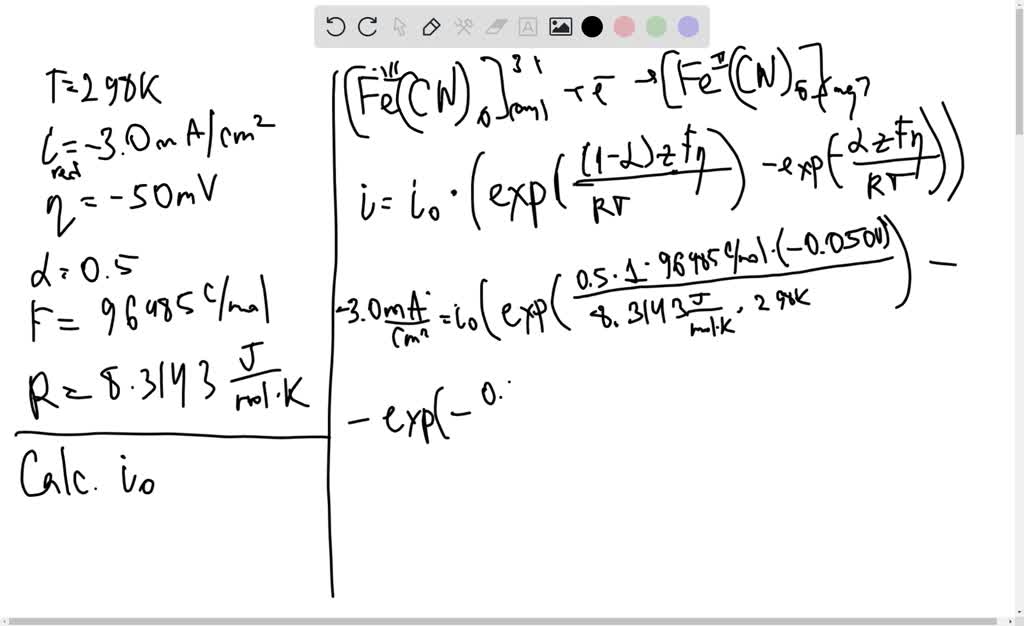
SOLVED: For a reaction at 298 K in which n = 1, α = 0.5, i0 = 2.0x10-6 Acm-2, and mass transfer effects can be ignored, calculate the cathodic current density flowing

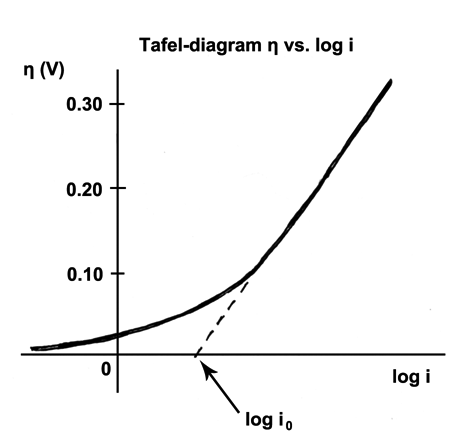
How to calculate exchange current density from Tafel plot for 2 chamber MFC? How other parameters like act potential/no of electrons are determined? | ResearchGate
Buffer pKa and Transport Govern the Concentration Overpotential in Electrochemical Oxygen Reduction at Neutral pH