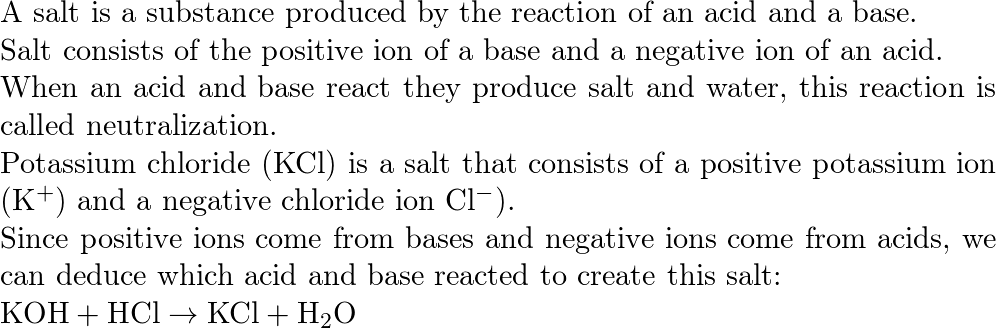Acid and Base Chemistry. Some Properties of Acids þ Produce H + (as H 3 O + ) ions in water (the hydronium ion is a hydrogen ion attached to a water molecule) - ppt download

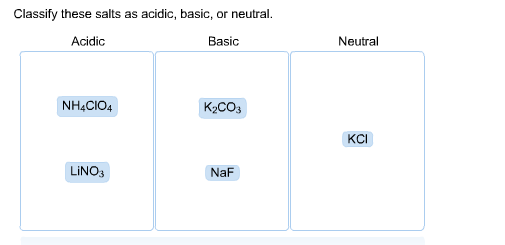
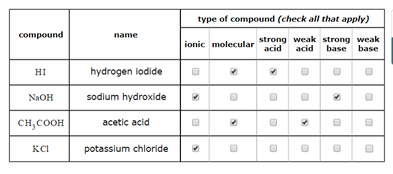
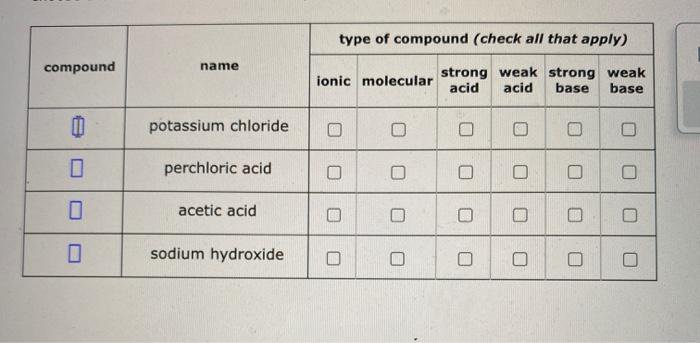
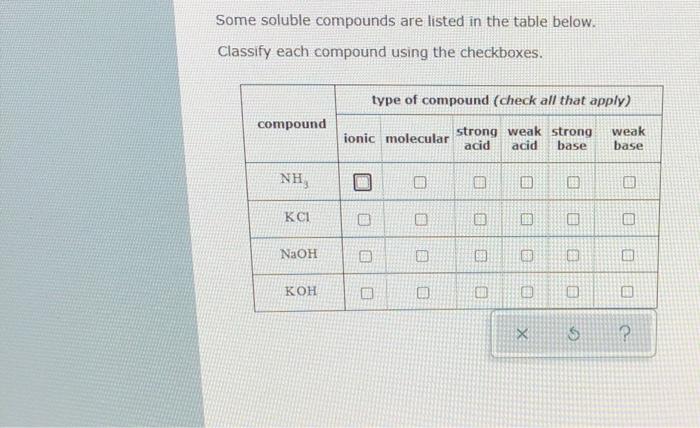
Classify these compounds as acid, base, salt, or other. NaOH, KCl, NH3, HNO3, HCOOH, CO2, NaBr, and CH3CH3? - Home Work Help - Learn CBSE Forum

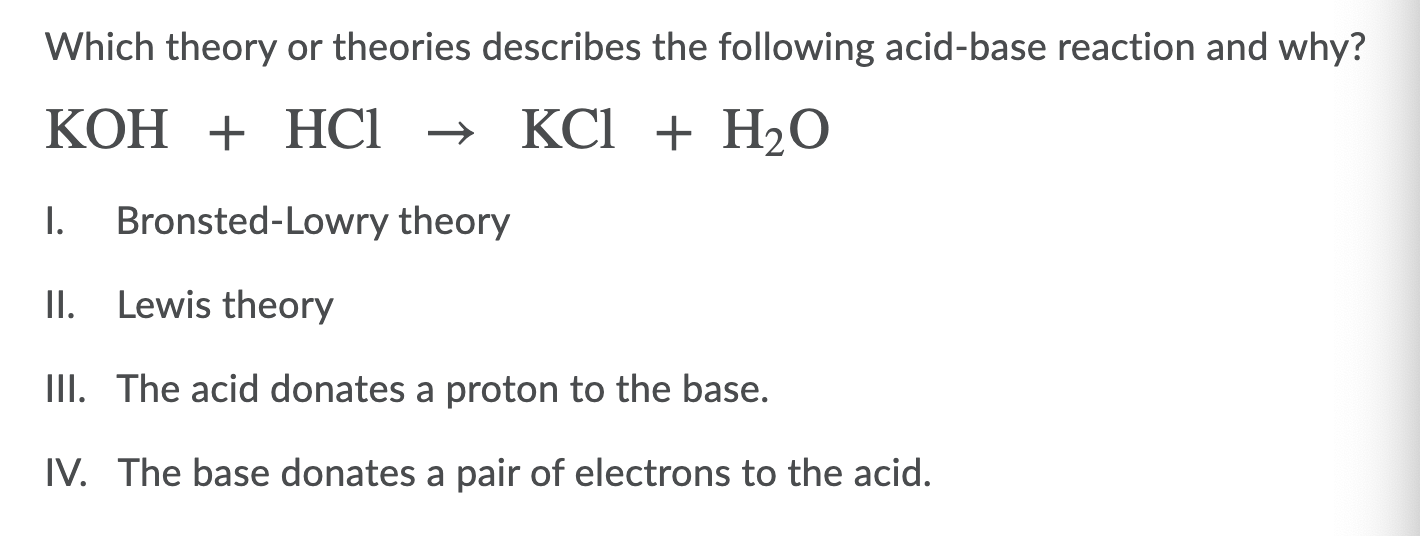
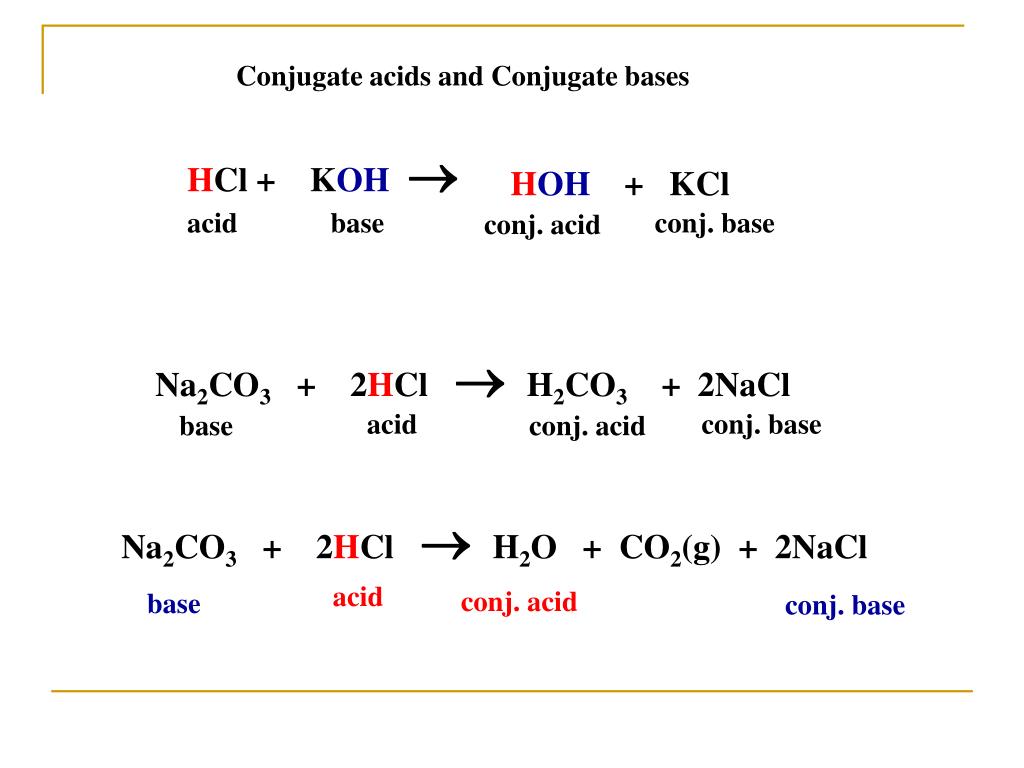
O8-#51-52 BASE ACID SALT WATER 51) KOH + HCl KCl + H 2 O COMBINE THE METAL K FROM THE BASE WITH THE NON METAL Cl FROM THE ACID TO

Balancing and writing acid base and acid carbonate equations renew - Interactive e-Worksheet - Quickworksheets
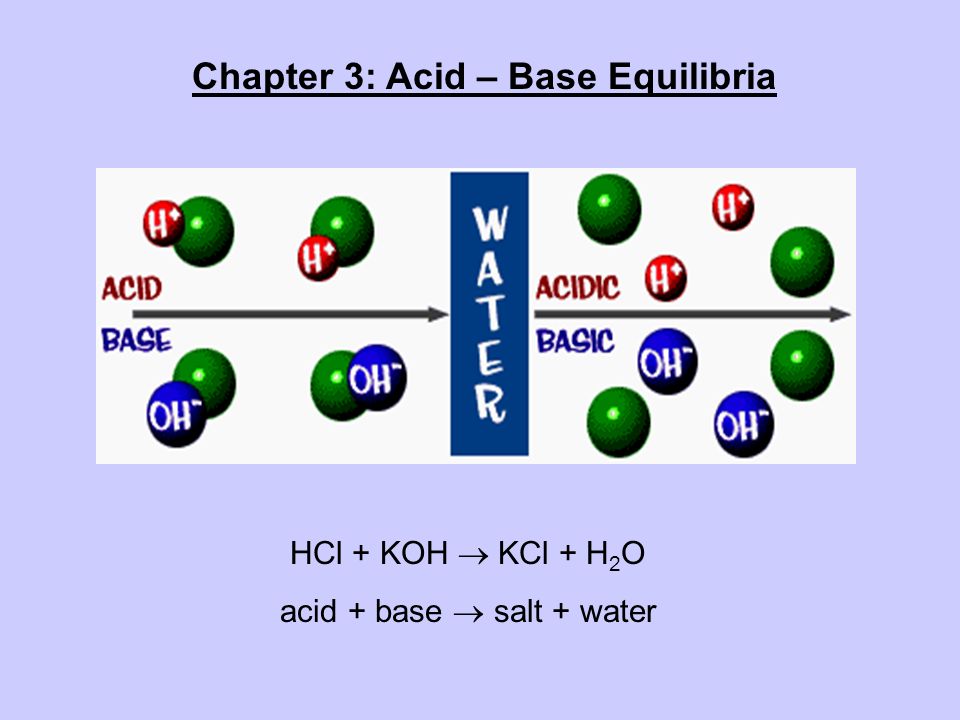
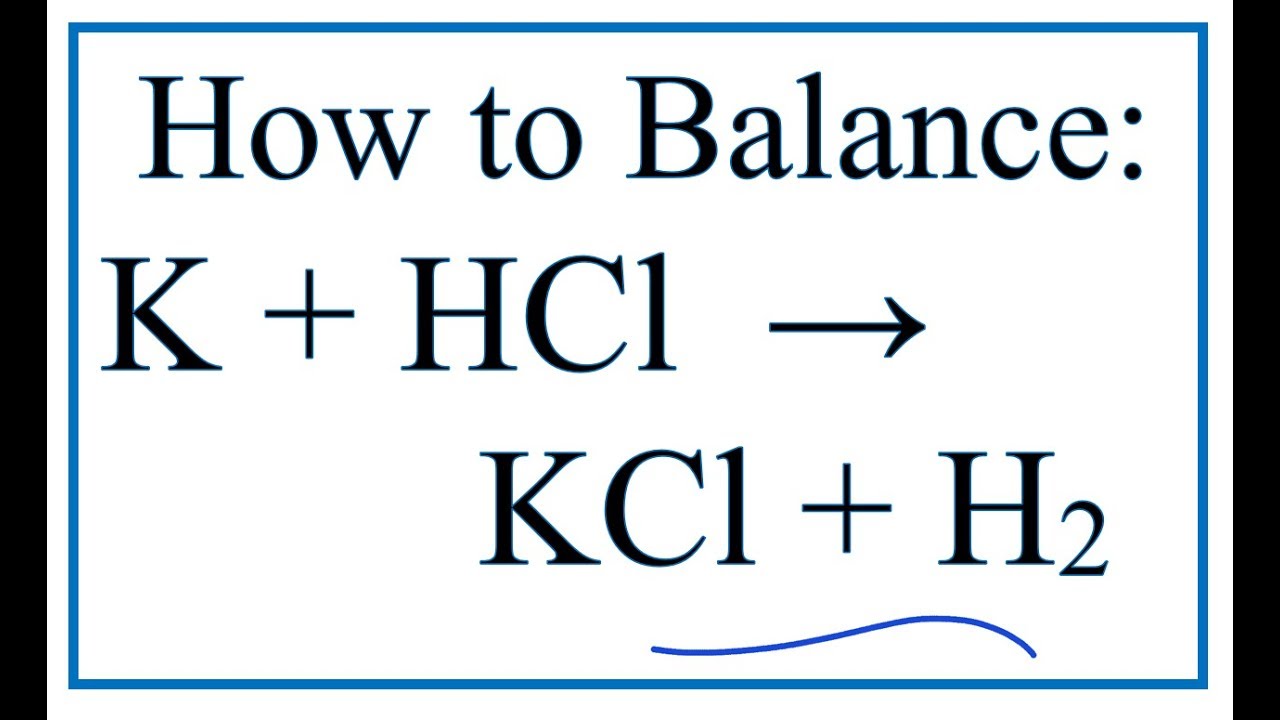
Chapter 3: Acid – Base Equilibria HCl + KOH KCl + H 2 O acid + base salt + water. - ppt download

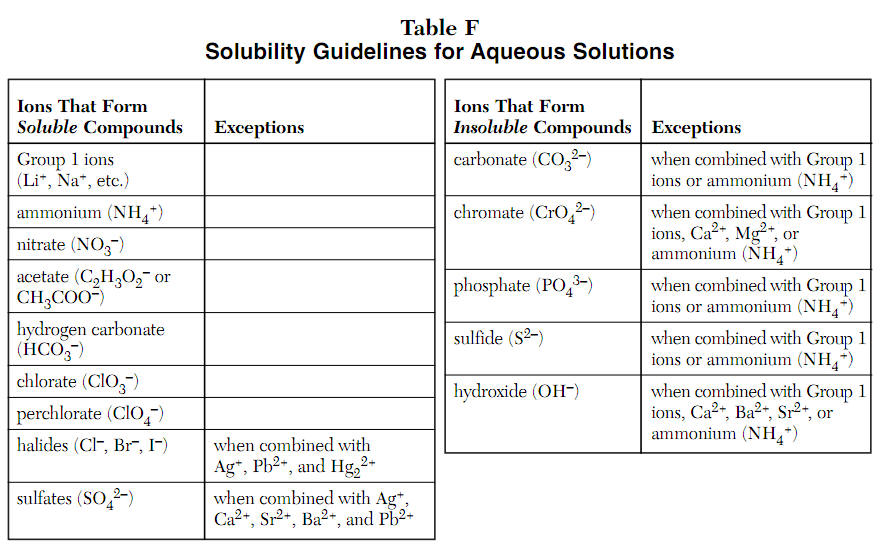
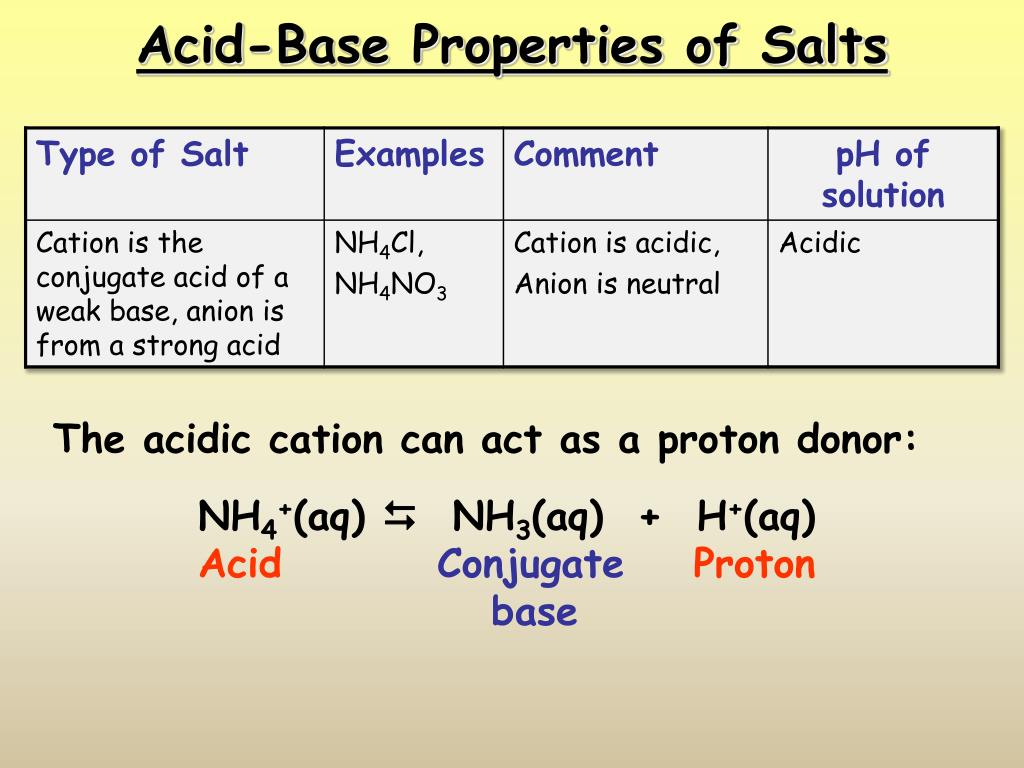
Acid-Base Properties of Salts. These salts simply dissociate in water: KCl(s) K + (aq) + Cl - (aq) - ppt download