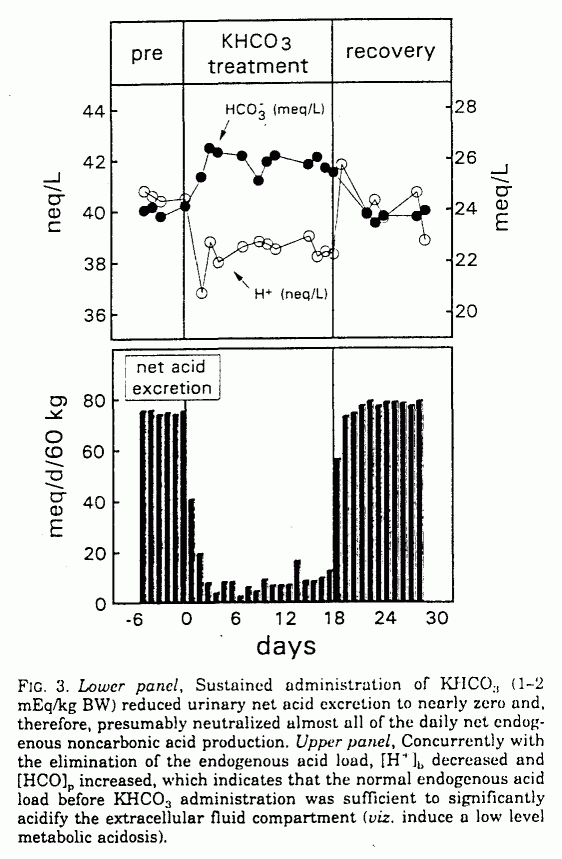
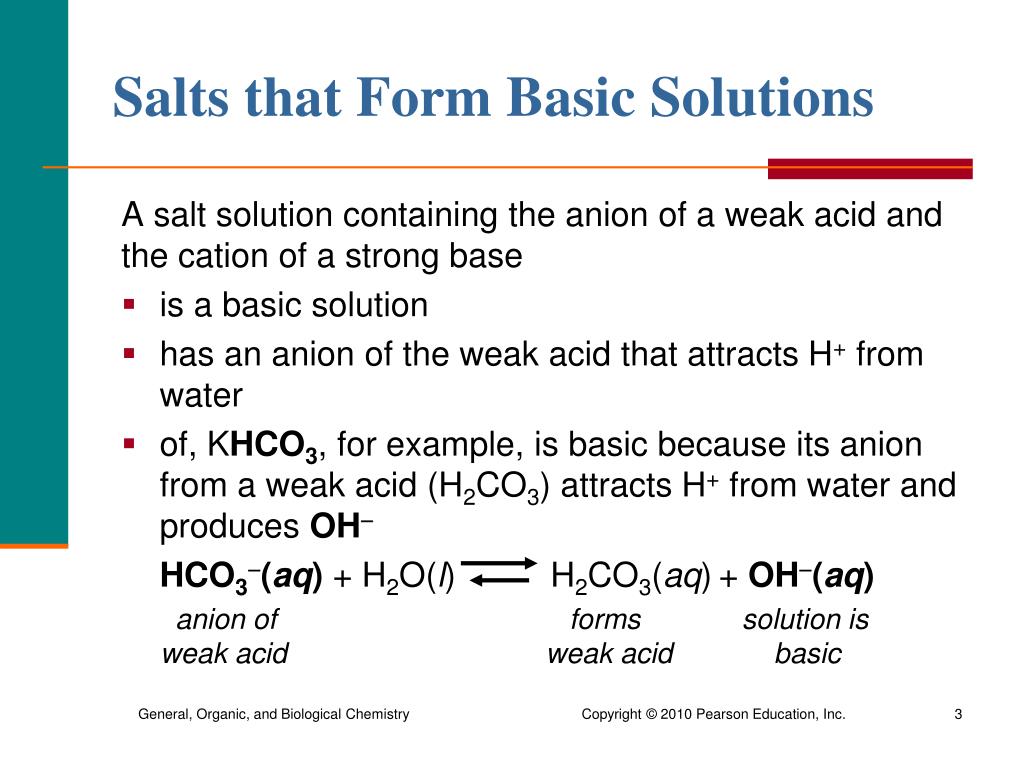
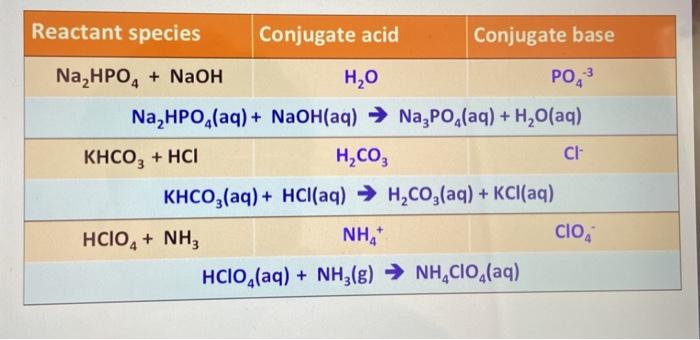
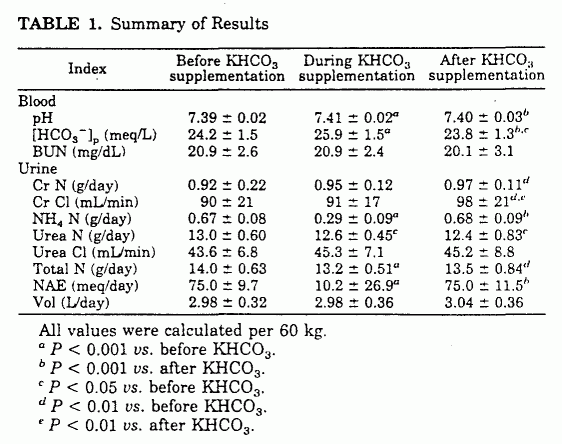
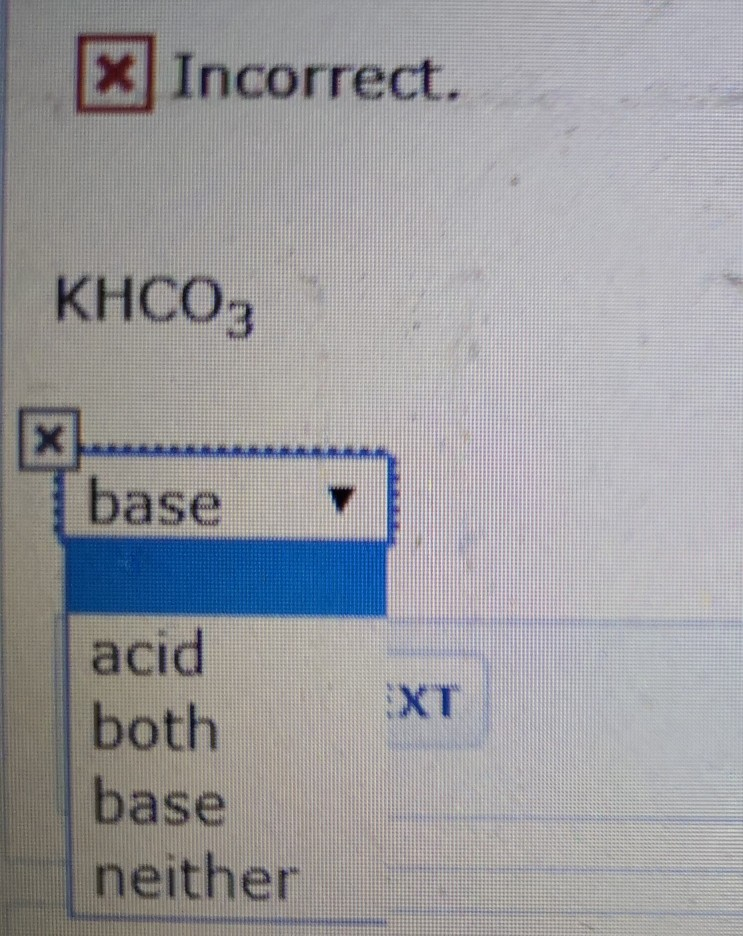
![Basic concepts: Acid-Base chemistry & pH 1.Recognizing acid/base and conjugate base/acid 2.Calculation of pH, pOH, [H 3 O + ], [OH - ] 3.Calculating pH. - ppt download Basic concepts: Acid-Base chemistry & pH 1.Recognizing acid/base and conjugate base/acid 2.Calculation of pH, pOH, [H 3 O + ], [OH - ] 3.Calculating pH. - ppt download](https://images.slideplayer.com/15/4572591/slides/slide_38.jpg)
Basic concepts: Acid-Base chemistry & pH 1.Recognizing acid/base and conjugate base/acid 2.Calculation of pH, pOH, [H 3 O + ], [OH - ] 3.Calculating pH. - ppt download

Measured rate of CO 2 gas (in slpm) emitted from the acid compartment... | Download Scientific Diagram
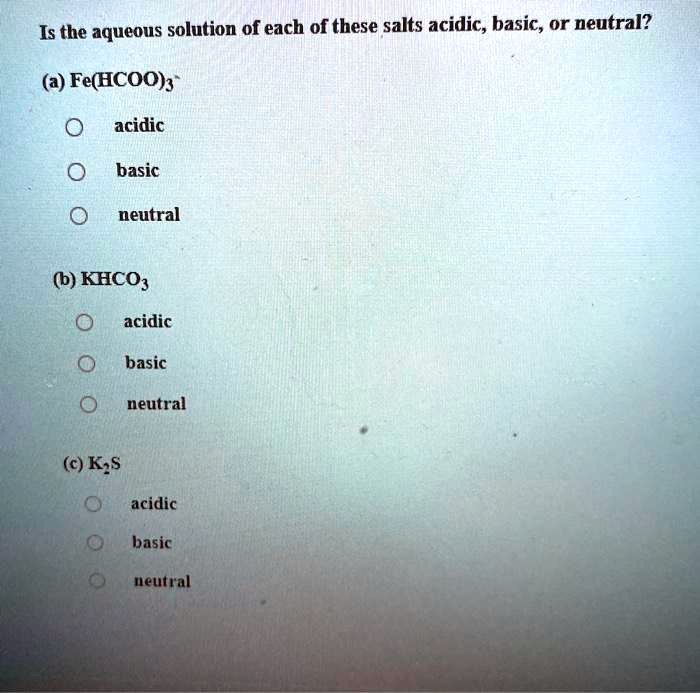
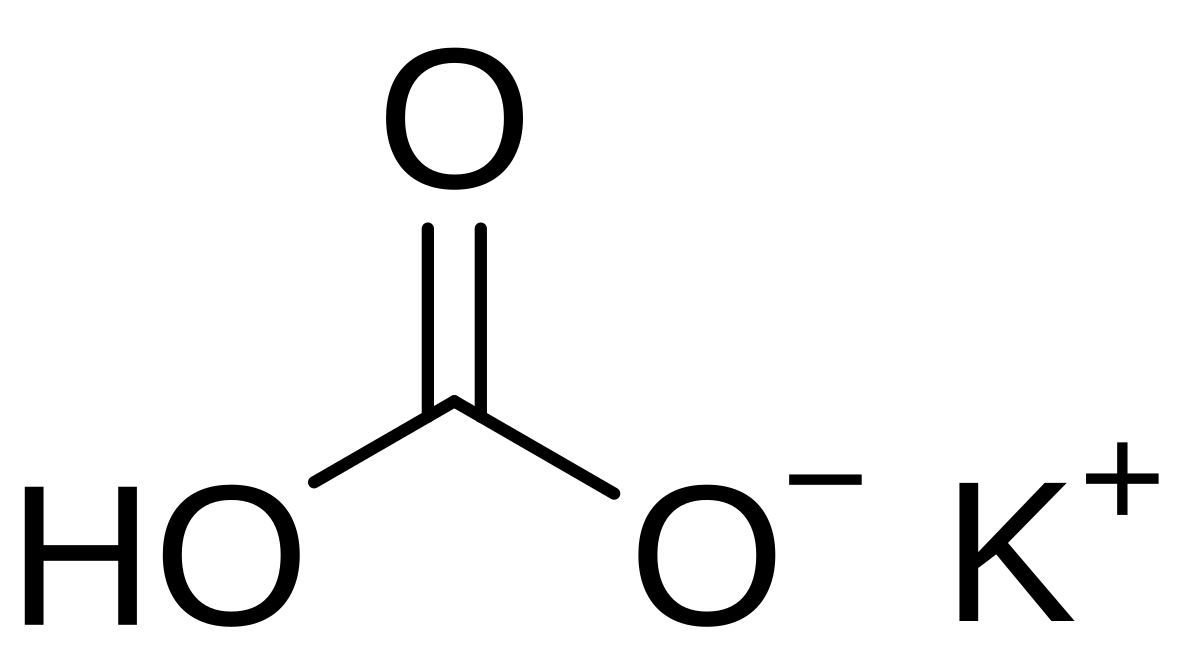
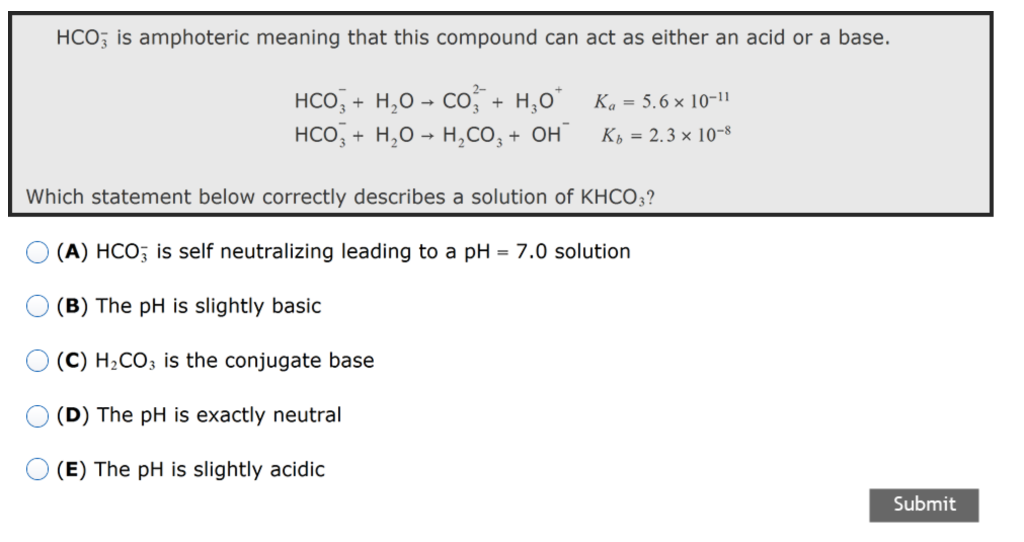
SOLVED: Is the aqueous solution of each of these salts acidic, basic, or neutral? (a) Fe(HCOO)s acidic basic neutral (b) KHCO acidic basic neutral (c) K;s acidic basic ueutral

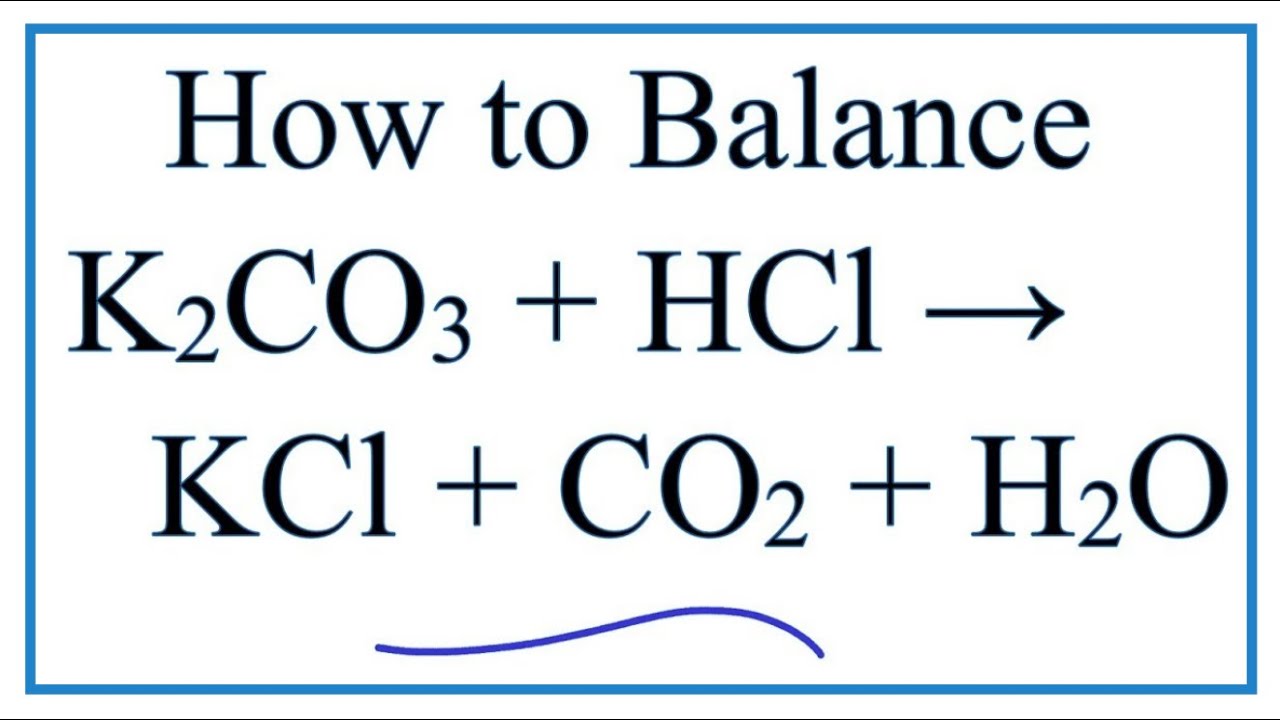
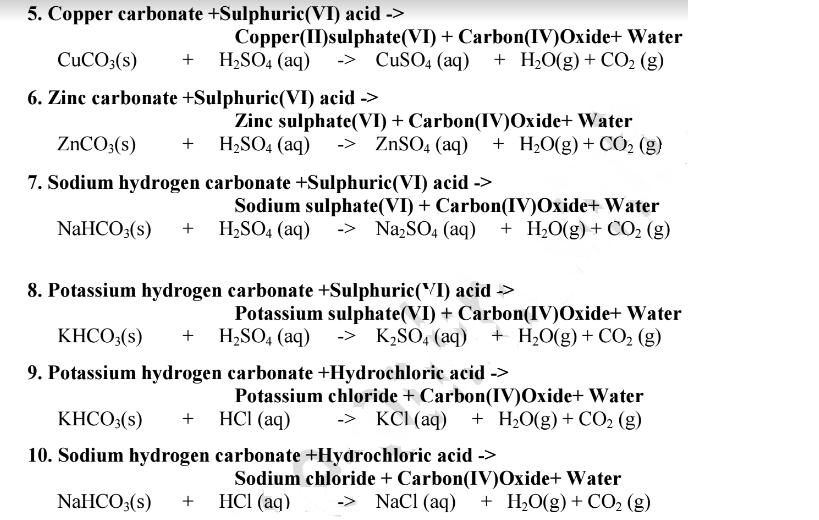
Difference Between Potassium Carbonate and Potassium Bicarbonate | Compare the Difference Between Similar Terms
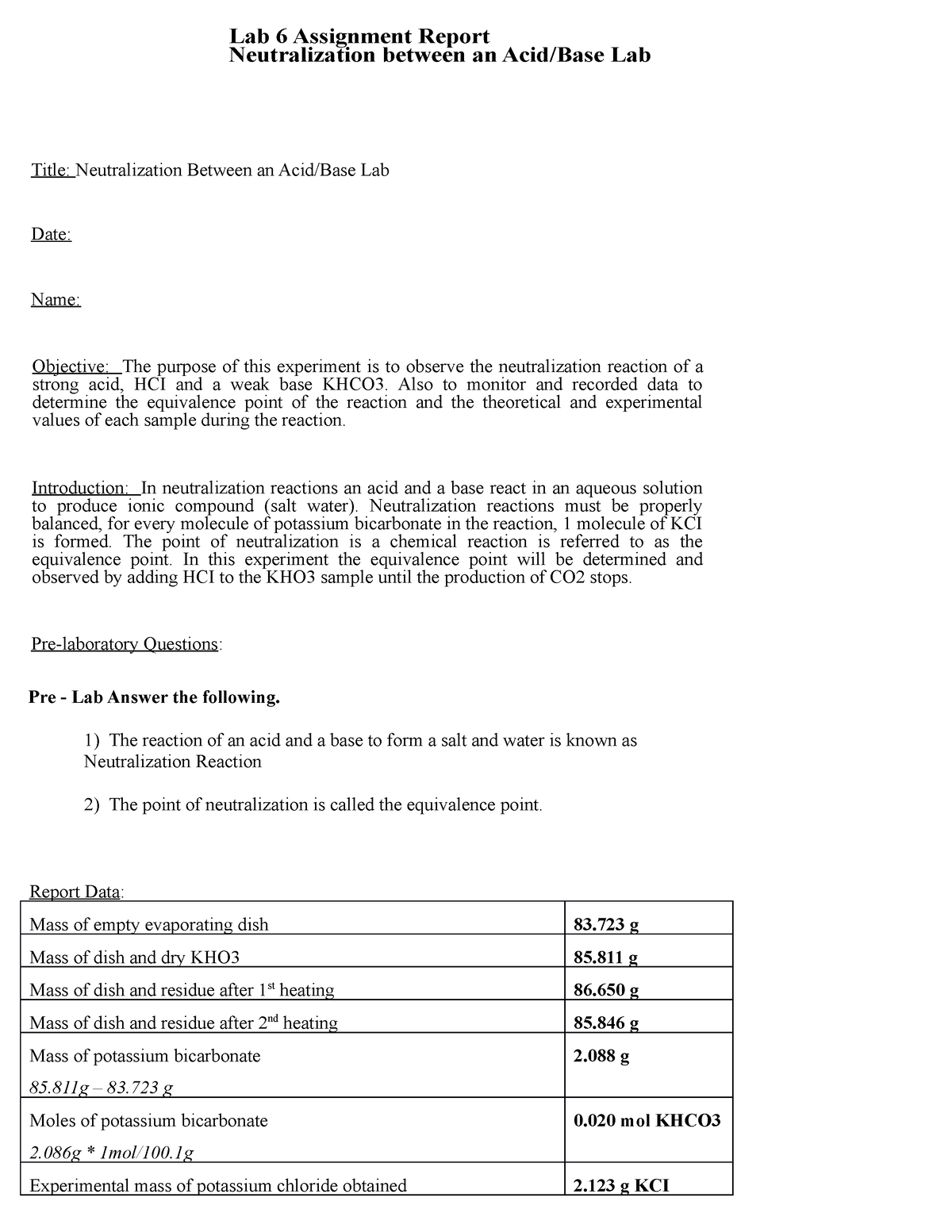
Lab 6 - Neutralization Between Acid/Base - Lab 6 Assignment Report Neutralization between an - StuDocu

The Importance of Acid–Base Equilibria in Bicarbonate Electrolytes for CO2 Electrochemical Reduction and CO Reoxidation Studied on Au(hkl) Electrodes | Langmuir