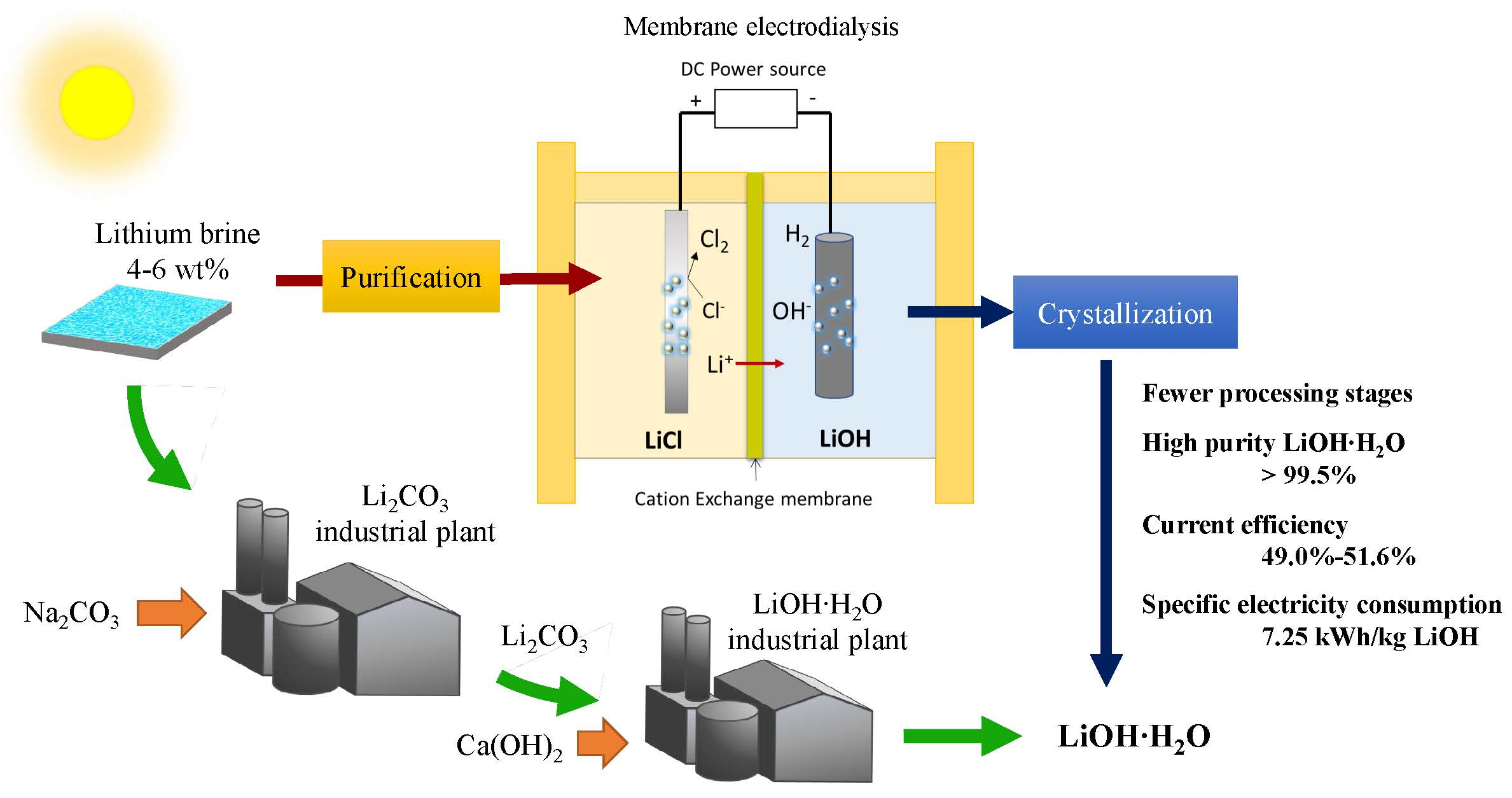
Membranes | Free Full-Text | Analysis of a Process for Producing Battery Grade Lithium Hydroxide by Membrane Electrodialysis
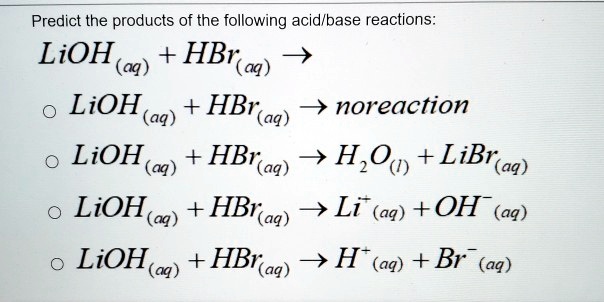
SOLVED: Predict the products of the following acid-base reactions: 1. LiOH (aq) + HBr (aq) â†' no reaction 2. LiOH (aq) + HBr (aq) â†' H2O (l) + LiBr (aq) 3. LiOH (

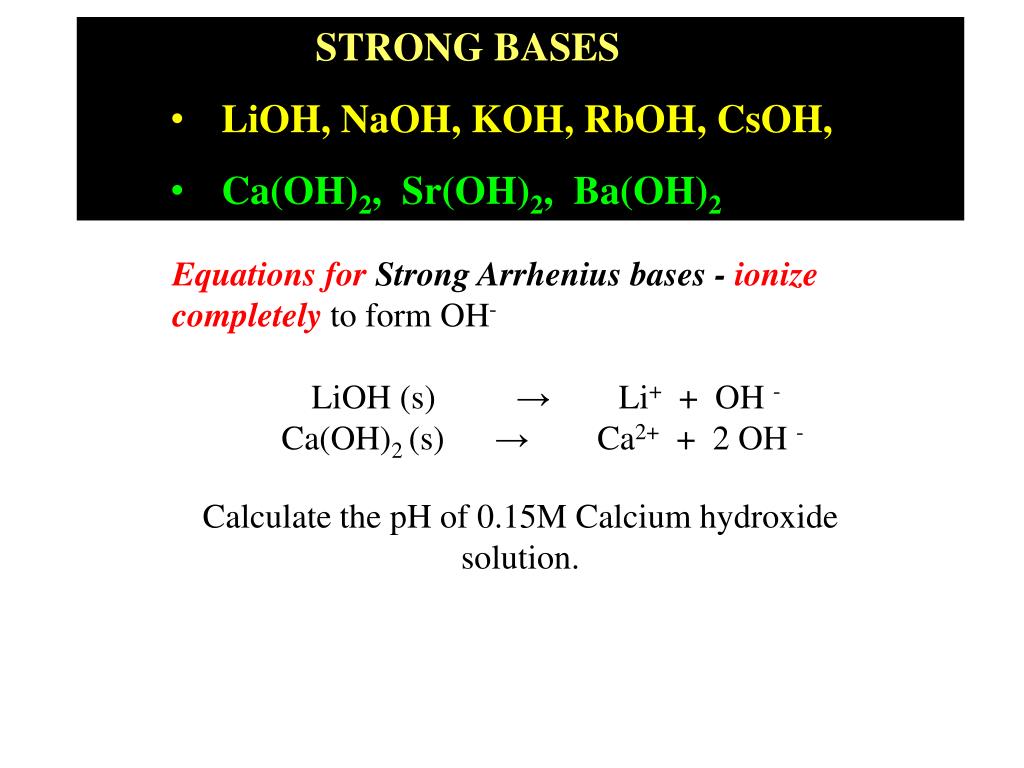
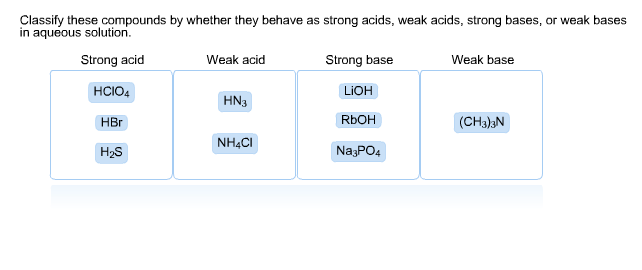
Equilibrium – Acids and Bases. Review of Acids and Bases Arrhenius Theory of Acids and Bases ▫An acid is a substance that dissociates in water to produce. - ppt download





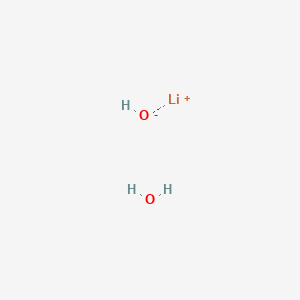
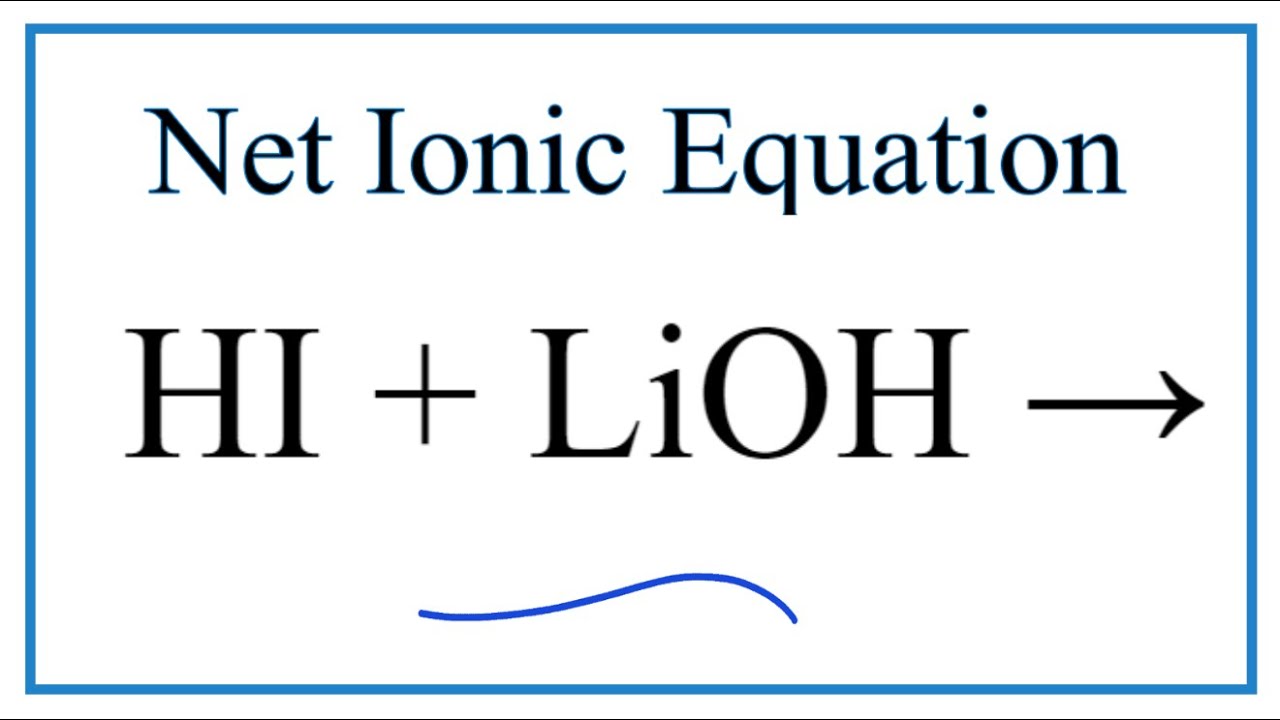







![PDF] LiOH.H2O as a catalyst for Knoevenagel and Gewald reactions | Semantic Scholar PDF] LiOH.H2O as a catalyst for Knoevenagel and Gewald reactions | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/345efc201af5c7998afa2f1e26d5b5916da3e9de/2-Table1-1.png)